Real-World Data From a Molecular Tumor Board: Improved Outcomes in Breast and Gynecologic Cancers Patients With Precision Medicine
- PMID: 35005995
- PMCID: PMC8769125
- DOI: 10.1200/PO.20.00508
Real-World Data From a Molecular Tumor Board: Improved Outcomes in Breast and Gynecologic Cancers Patients With Precision Medicine
Abstract
Purpose: Next-generation sequencing is increasingly used in gynecologic and breast cancers. Multidisciplinary Molecular Tumor Board (MTB) may guide matched therapy; however, outcome data are limited. We evaluate the effect of the degree of matching of tumors to treatment as well as compliance to MTB recommendations on outcomes.
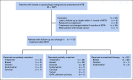
Methods: Overall, 164 patients with consecutive gynecologic and breast cancers presented at MTB were assessed for clinicopathologic data, next-generation sequencing results, MTB recommendations, therapy received, and outcomes. Matching score (MS), defined as percentage of alterations targeted by treatment over total pathogenic alterations, and compliance to MTB recommendations were analyzed in context of oncologic outcomes.
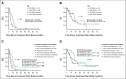
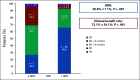
Results: Altogether, 113 women were evaluable for treatment after MTB; 54% received matched therapy. Patients with MS ≥ 40% had higher overall response rate (30.8% v 7.1%; P = .001), progression-free survival (PFS; hazard ratio [HR] 0.51; 95% CI, 0.31 to 0.85; P = .002), and a trend toward improved overall survival (HR 0.64; 95% CI, 0.34 to 1.25; P = .082) in univariate analysis. The PFS advantage remained significant in multivariate analysis (HR 0.5; 95% CI, 0.3 to 0.8; P = .006). Higher MTB recommendation compliance was significantly associated with improved median PFS (9.0 months for complete; 6.0 months for partial; 4.0 months for no compliance; P = .004) and overall survival (17.1 months complete; 17.8 months partial; 10.8 months none; P = .046). Completely MTB-compliant patients had higher MS (P < .001). In multivariate analysis comparing all versus none MTB compliance, overall response (HR 9.5; 95% CI, 2.6 to 35.0; P = .001) and clinical benefit (HR 8.8; 95% CI, 2.4 to 33.2; P = .001) rates were significantly improved with higher compliance.
Conclusion: Compliance to MTB recommendations resulted in higher degrees of matched therapy and correlates with improved outcomes in patients with gynecologic and breast cancers.
Conflict of interest statement
Figures
References
-
- Schwaederle M, Zhao M, Lee JJ, et al. : Association of biomarker-based treatment strategies with response rates and progression-free survival in refractory malignant neoplasms: A meta-analysis. JAMA Oncol 2:1452-1459, 2016 - PubMed
-
- Shatsky R, Parker BA, Bui NQ, et al. : Next-generation sequencing of tissue and circulating tumor DNA: The UC San Diego Moores Center for personalized cancer therapy experience with breast malignancies. Mol Cancer Ther 18:1001-1011, 2019 - PubMed

