Safety and Effectiveness of Long-term Intravenous Administration of Edaravone for Treatment of Patients With Amyotrophic Lateral Sclerosis
- PMID: 35006266
- PMCID: PMC8749709
- DOI: 10.1001/jamaneurol.2021.4893
Safety and Effectiveness of Long-term Intravenous Administration of Edaravone for Treatment of Patients With Amyotrophic Lateral Sclerosis
Erratum in
-
Errors in Results in Abstract and Text and in Table 1.JAMA Neurol. 2022 Jul 1;79(7):727. doi: 10.1001/jamaneurol.2022.1581. JAMA Neurol. 2022. PMID: 35696158 Free PMC article. No abstract available.
Abstract
Importance: Intravenous edaravone is approved as a disease-modifying drug for patients with amyotrophic lateral sclerosis (ALS), but evidence for efficacy is limited to short-term beneficial effects shown in the MCI186-ALS19 study in a subpopulation in which efficacy was expected.
Objective: To evaluate the long-term safety and effectiveness of intravenous edaravone therapy for patients with ALS in a real-world clinical setting.
Design, setting, and participants: Multicenter, propensity score-matched cohort study conducted between June 2017 and March 2020 at 12 academic ALS referral centers associated with the German Motor Neuron Disease Network. Of 1440 patients screened, 738 were included in propensity score matching. Final analyses included 324 patients with ALS comprising 194 patients who started intravenous edaravone treatment (141 received ≥4 consecutive treatment cycles; 130 matched) and 130 propensity score-matched patients with ALS receiving standard therapy. All patients had probable or definite ALS according to the El Escorial criteria, with disease onset between December 2012 and April 2019. Subgroups were defined by applying the MCI186-ALS19 study inclusion criteria to evaluate whether patients would have been considered eligible (EFAS) or ineligible (non-EFAS).
Exposures: Intravenous edaravone plus riluzole vs riluzole only.
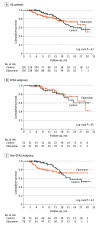
Main outcomes and measures: Patient characteristics and systematic safety assessment for patients who received at least 1 dose of intravenous edaravone. Effectiveness assessment of edaravone was conducted among patients who received at least 4 treatment cycles compared with propensity score-matched patients with ALS who received only standard therapy. Primary outcome was disease progression measured by decrease in the ALS Functional Rating Scale-Revised (ALSFRS-R) score. Secondary outcomes were survival probability, time to ventilation, and change in disease progression before vs during treatment. To account for the matched design, patients receiving edaravone and their corresponding matched controls were regarded as related samples in disease progression analyses; stratification for propensity score quintiles was used for survival probability and time to ventilation analyses.
Results: A total of 194 patients started intravenous edaravone treatment; 125 (64%) were male, and the median age was 57.5 years (IQR, 50.7-63.8 years). Potential adverse effects were observed in 30 cases (16%), most notably infections at infusion sites and allergic reactions. Disease progression among 116 patients treated for a median of 13.9 months (IQR, 8.9-13.9 months) with edaravone did not differ from 116 patients treated for a median of 11.2 months (IQR, 6.4-20.0 months) with standard therapy (ALSFRS-R points/month, -0.91 [95% CI, -0.69 to -1.07] vs -0.85 [95% CI, -0.66 to -0.99]; P = .37). No significant differences were observed in the secondary end points of survival probability, time to ventilation, and change in disease progression. Similarly, outcomes between patients treated with edaravone and matched patients did not differ within the EFAS and non-EFAS subgroups.
Conclusions and relevance: This cohort study using propensity score matching found that, although long-term intravenous edaravone therapy for patients with ALS was feasible and mainly well tolerated, it was not associated with any disease-modifying benefit. Intravenous edaravone may not provide a clinically relevant additional benefit compared with standard therapy alone.
Conflict of interest statement
Figures


Comment in
-
Unintended Consequences of Approving Unproven Treatments-Hope, Hype, or Harm?JAMA Neurol. 2022 Feb 1;79(2):117-118. doi: 10.1001/jamaneurol.2021.4193. JAMA Neurol. 2022. PMID: 35006259 No abstract available.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

