Development of a novel TLR8 agonist for cancer immunotherapy
- PMID: 35006413
- PMCID: PMC8607422
- DOI: 10.1186/s43556-020-00007-y
Development of a novel TLR8 agonist for cancer immunotherapy
Erratum in
-
Correction to: Development of a novel TLR8 agonist for cancer immunotherapy.Mol Biomed. 2021 May 21;2(1):16. doi: 10.1186/s43556-021-00042-3. Mol Biomed. 2021. PMID: 35006462 Free PMC article. No abstract available.
Abstract
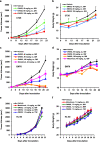
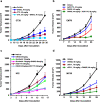
Toll-like receptors (TLRs) are a family of proteins that recognize pathogen associated molecular patterns (PAMPs). Their primary function is to activate innate immune responses while also involved in facilitating adaptive immune responses. Different TLRs exert distinct functions by activating varied immune cascades. Several TLRs are being pursued as cancer drug targets. We discovered a novel, highly potent and selective small molecule TLR8 agonist DN052. DN052 exhibited strong in vitro cellular activity with EC50 at 6.7 nM and was highly selective for TLR8 over other TLRs including TLR4, 7 and 9. DN052 displayed excellent in vitro ADMET and in vivo PK profiles. DN052 potently inhibited tumor growth as a single agent. Moreover, combination of DN052 with the immune checkpoint inhibitor, selected targeted therapeutics or chemotherapeutic drugs further enhanced efficacy of single agents. Mechanistically, treatment with DN052 resulted in strong induction of pro-inflammatory cytokines in ex vivo human PBMC assay and in vivo monkey study. GLP toxicity studies in rats and monkeys demonstrated favorable safety profile. This led to the advancement of DN052 into phase 1 clinical trials.
Keywords: Cancer; Immunotherapy; Innate immunity; TLR8.
© 2020. The Author(s).
Conflict of interest statement
YW, HY and DG have patent 10669252 (Benzazepine derivative, preparation method, pharmaceuticalcomposition and use thereof). YW, HY, HL, SZ, YZ, PZ, XL, XS, LW, GF, YG, PW and DG are the employees of Shanghai Denovo Pharmatech Co., Ltd.. This work was supported by Shanghai Denovo Pharmatech Co., Ltd..
Figures


Similar articles
-
Targeting toll-like receptor 7/8 for immunotherapy: recent advances and prospectives.Biomark Res. 2022 Dec 7;10(1):89. doi: 10.1186/s40364-022-00436-7. Biomark Res. 2022. PMID: 36476317 Free PMC article. Review.
-
Toll-like receptor 8 agonist nanoparticles mimic immunomodulating effects of the live BCG vaccine and enhance neonatal innate and adaptive immune responses.J Allergy Clin Immunol. 2017 Nov;140(5):1339-1350. doi: 10.1016/j.jaci.2016.12.985. Epub 2017 Mar 23. J Allergy Clin Immunol. 2017. PMID: 28343701 Free PMC article.
-
The functional effects of physical interactions among Toll-like receptors 7, 8, and 9.J Biol Chem. 2006 Dec 8;281(49):37427-34. doi: 10.1074/jbc.M605311200. Epub 2006 Oct 13. J Biol Chem. 2006. PMID: 17040905
-
The ultra-potent and selective TLR8 agonist VTX-294 activates human newborn and adult leukocytes.PLoS One. 2013;8(3):e58164. doi: 10.1371/journal.pone.0058164. Epub 2013 Mar 4. PLoS One. 2013. PMID: 23483986 Free PMC article.
-
TLRs as a Promise Target Along With Immune Checkpoint Against Gastric Cancer.Front Cell Dev Biol. 2021 Jan 5;8:611444. doi: 10.3389/fcell.2020.611444. eCollection 2020. Front Cell Dev Biol. 2021. PMID: 33469538 Free PMC article. Review.
Cited by
-
Correction to: Development of a novel TLR8 agonist for cancer immunotherapy.Mol Biomed. 2021 May 21;2(1):16. doi: 10.1186/s43556-021-00042-3. Mol Biomed. 2021. PMID: 35006462 Free PMC article. No abstract available.
-
Immunotherapy in Ovarian Cancer: Thinking Beyond PD-1/PD-L1.Front Oncol. 2021 Dec 13;11:795547. doi: 10.3389/fonc.2021.795547. eCollection 2021. Front Oncol. 2021. PMID: 34966689 Free PMC article. Review.
-
Senescent T Cells: The Silent Culprit in Acute Myeloid Leukemia Progression?Int J Mol Sci. 2024 Nov 22;25(23):12550. doi: 10.3390/ijms252312550. Int J Mol Sci. 2024. PMID: 39684260 Free PMC article. Review.
-
Targeting toll-like receptor 7/8 for immunotherapy: recent advances and prospectives.Biomark Res. 2022 Dec 7;10(1):89. doi: 10.1186/s40364-022-00436-7. Biomark Res. 2022. PMID: 36476317 Free PMC article. Review.
References
-
- Bourquin C, Pommier A, Hotz C. Harnessing the immune system to fight cancer with toll-like receptor and RIG-I-like receptor agonists. Pharmacol Res. 2019;104192. 10.1016/j.phrs.2019.03.001. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
