RAD50 deficiency is a predictor of platinum sensitivity in sporadic epithelial ovarian cancers
- PMID: 35006434
- PMCID: PMC8607373
- DOI: 10.1186/s43556-020-00023-y
RAD50 deficiency is a predictor of platinum sensitivity in sporadic epithelial ovarian cancers
Abstract
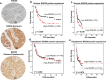
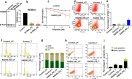
Intrinsic or acquired resistance seriously limits the use of platinating agents in advanced epithelial ovarian cancers. Increased DNA repair capacity is a key route to platinum resistance. RAD50 is a critical component of the MRN complex, a 'first responder' to DNA damage and essential for the repair of DSBs and stalled replication forks. We hypothesised a role for RAD50 in ovarian cancer pathogenesis and therapeutics. Clinicopathological significance of RAD50 expression was evaluated in clinical cohorts of ovarian cancer at the protein level (n = 331) and at the transcriptomic level (n = 1259). Sub-cellular localization of RAD50 at baseline and following cisplatin therapy was tested in platinum resistant (A2780cis, PEO4) and sensitive (A2780, PEO1) ovarian cancer cells. RAD50 was depleted and cisplatin sensitivity was investigated in A2780cis and PEO4 cells. RAD50 deficiency was associated with better progression free survival (PFS) at the protein (p = 0.006) and transcriptomic level (p < 0.001). Basal level of RAD50 was higher in platinum resistant cells. Following cisplatin treatment, increased nuclear localization of RAD50 was evident in A2780cis and PEO4 compared to A2780 and PEO1 cells. RAD50 depletion using siRNAs in A2780cis and PEO4 cells increased cisplatin cytotoxicity, which was associated with accumulation of DSBs, S-phase cell cycle arrest and increased apoptosis. We provide evidence that RAD50 deficiency is a predictor of platinum sensitivity. RAD50 expression-based stratification and personalization could be viable clinical strategy in ovarian cancers.
Keywords: DNA repair; Ovarian cancer; Platinum therapy; Predictive biomarker; RAD50.
© 2020. The Author(s).
Conflict of interest statement
The authors disclose no potential conflicts of interest.
Figures



References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
