Cancer metabolism and intervention therapy
- PMID: 35006438
- PMCID: PMC8607959
- DOI: 10.1186/s43556-020-00012-1
Cancer metabolism and intervention therapy
Abstract
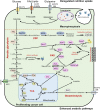
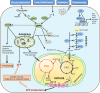
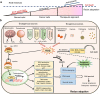
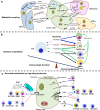
Metabolic reprogramming with heterogeneity is a hallmark of cancer and is at the basis of malignant behaviors. It supports the proliferation and metastasis of tumor cells according to the low nutrition and hypoxic microenvironment. Tumor cells frantically grab energy sources (such as glucose, fatty acids, and glutamine) from different pathways to produce a variety of biomass to meet their material needs via enhanced synthetic pathways, including aerobic glycolysis, glutaminolysis, fatty acid synthesis (FAS), and pentose phosphate pathway (PPP). To survive from stress conditions (e.g., metastasis, irradiation, or chemotherapy), tumor cells have to reprogram their metabolism from biomass production towards the generation of abundant adenosine triphosphate (ATP) and antioxidants. In addition, cancer cells remodel the microenvironment through metabolites, promoting an immunosuppressive microenvironment. Herein, we discuss how the metabolism is reprogrammed in cancer cells and how the tumor microenvironment is educated via the metabolic products. We also highlight potential metabolic targets for cancer therapies.
Keywords: Cancer; Heterogeneity; Metabolic reprogramming; Targeted therapy; Tumor microenvironment.
© 2021. The Author(s).
Conflict of interest statement
The authors have declared that no competing interest exists.
Figures
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
