Cerebellar long-term depression and auto-immune target of auto-antibodies: the concept of LTDpathies
- PMID: 35006439
- PMCID: PMC8607360
- DOI: 10.1186/s43556-020-00024-x
Cerebellar long-term depression and auto-immune target of auto-antibodies: the concept of LTDpathies
Abstract
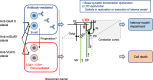
There is general agreement that auto-antibodies against ion channels and synaptic machinery proteins can induce limbic encephalitis. In immune-mediated cerebellar ataxias (IMCAs), various synaptic proteins, such as GAD65, voltage-gated Ca channel (VGCC), metabotropic glutamate receptor type 1 (mGluR1), and glutamate receptor delta (GluR delta) are auto-immune targets. Among them, the pathophysiological mechanisms underlying anti-VGCC, anti-mGluR1, and anti-GluR delta antibodies remain unclear. Despite divergent auto-immune and clinical profiles, these subtypes show common clinical features of good prognosis with no or mild cerebellar atrophy in non-paraneoplastic syndrome. The favorable prognosis reflects functional cerebellar disorders without neuronal death. Interestingly, these autoantigens are all involved in molecular cascades for induction of long-term depression (LTD) of synaptic transmissions between parallel fibers (PFs) and Purkinje cells (PCs), a crucial mechanism of synaptic plasticity in the cerebellum. We suggest that anti-VGCC, anti-mGluR1, and anti-GluR delta Abs-associated cerebellar ataxias share one common pathophysiological mechanism: a deregulation in PF-PC LTD, which results in impairment of restoration or maintenance of the internal model and triggers cerebellar ataxias. The novel concept of LTDpathies could lead to improvements in clinical management and treatment of cerebellar patients who show these antibodies.
Keywords: Anti-GluR delta antibody; Anti-VGCC antibody; Anti-mGluR antibody; Cerebellar ataxias; Immune-mediated cerebellar ataxias; Long-term depression.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Advances in the Pathogenesis of Auto-antibody-Induced Cerebellar Synaptopathies.Cerebellum. 2023 Feb;22(1):129-147. doi: 10.1007/s12311-021-01359-z. Epub 2022 Jan 22. Cerebellum. 2023. PMID: 35064896 Free PMC article. Review.
-
The Clinical Concept of LTDpathy: Is Dysregulated LTD Responsible for Prodromal Cerebellar Symptoms?Brain Sci. 2022 Feb 24;12(3):303. doi: 10.3390/brainsci12030303. Brain Sci. 2022. PMID: 35326260 Free PMC article.
-
Fundamental Mechanisms of Autoantibody-Induced Impairments on Ion Channels and Synapses in Immune-Mediated Cerebellar Ataxias.Int J Mol Sci. 2020 Jul 13;21(14):4936. doi: 10.3390/ijms21144936. Int J Mol Sci. 2020. PMID: 32668612 Free PMC article. Review.
-
Progressive impairment of cerebellar mGluR signalling and its therapeutic potential for cerebellar ataxia in spinocerebellar ataxia type 1 model mice.J Physiol. 2017 Jan 1;595(1):141-164. doi: 10.1113/JP272950. Epub 2016 Sep 15. J Physiol. 2017. PMID: 27440721 Free PMC article.
-
Long-Term Depression of Intrinsic Excitability Accompanied by Synaptic Depression in Cerebellar Purkinje Cells.J Neurosci. 2017 Jun 7;37(23):5659-5669. doi: 10.1523/JNEUROSCI.3464-16.2017. Epub 2017 May 11. J Neurosci. 2017. PMID: 28495974 Free PMC article.
Cited by
-
The Emerging Key Role of the mGluR1-PKCγ Signaling Pathway in the Pathogenesis of Spinocerebellar Ataxias: A Neurodevelopmental Viewpoint.Int J Mol Sci. 2022 Aug 15;23(16):9169. doi: 10.3390/ijms23169169. Int J Mol Sci. 2022. PMID: 36012439 Free PMC article. Review.
-
Advances in the Pathogenesis of Auto-antibody-Induced Cerebellar Synaptopathies.Cerebellum. 2023 Feb;22(1):129-147. doi: 10.1007/s12311-021-01359-z. Epub 2022 Jan 22. Cerebellum. 2023. PMID: 35064896 Free PMC article. Review.
-
The Clinical Concept of LTDpathy: Is Dysregulated LTD Responsible for Prodromal Cerebellar Symptoms?Brain Sci. 2022 Feb 24;12(3):303. doi: 10.3390/brainsci12030303. Brain Sci. 2022. PMID: 35326260 Free PMC article.
-
Different Numbers of Conjunctive Stimuli Induce LTP or LTD in Mouse Cerebellar Purkinje Cell.Cerebellum. 2024 Dec;23(6):2297-2307. doi: 10.1007/s12311-024-01726-6. Epub 2024 Aug 3. Cerebellum. 2024. PMID: 39096432 Free PMC article.
-
Physiology of Cerebellar Reserve: Redundancy and Plasticity of a Modular Machine.Int J Mol Sci. 2021 Apr 30;22(9):4777. doi: 10.3390/ijms22094777. Int J Mol Sci. 2021. PMID: 33946358 Free PMC article. Review.
References
Publication types
LinkOut - more resources
Full Text Sources
