Mibefradil alters intracellular calcium concentration by activation of phospholipase C and IP3 receptor function
- PMID: 35006468
- PMCID: PMC8607413
- DOI: 10.1186/s43556-021-00037-0
Mibefradil alters intracellular calcium concentration by activation of phospholipase C and IP3 receptor function
Abstract
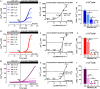
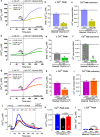
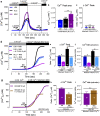
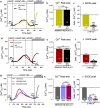
Mibefradil is a tetralol derivative originally developed as an antagonist of T-type voltage-gated calcium (Ca2+) channels to treat hypertension when used at nanomolar dosage. More recently, its therapeutic application in hypertension has declined and has been instead repurposed as a treatment of cancer cell proliferation and solid tumor growth. Beyond its function as a Cav blocker, the micromolar concentration of mibefradil can stimulate a rise in [Ca2+]cyt although the mechanism is poorly known. The chanzyme TRPM7 (transient receptor potential melastanin 7), the release of intracellular Ca2+ pools, and Ca2+ influx by ORAI channels have been associated with the increase in [Ca2+]cyt triggered by mibefradil. This study aims to investigate the cellular targets and pathways associated with mibefradil's effect on [Ca2+]cyt. To address these questions, we monitored changes in [Ca2+]cyt in the specialized mouse epithelial cells (LS8 and ALC) and the widely used HEK-293 cells by stimulating these cells with mibefradil (0.1 μM to 100 μM). We show that mibefradil elicits an increase in [Ca2+]cyt at concentrations above 10 μM (IC50 around 50 μM) and a fast Ca2+ increase capacity at 100 μM. We found that inhibiting IP3 receptors, depleting the ER-Ca2+ stores, or blocking phospholipase C (PLC), significantly decreased the capacity of mibefradil to elevate [Ca2+]cyt. Moreover, the transient application of 100 μM mibefradil triggered Ca2+ influx by store-operated Ca2+ entry (SOCE) mediated by the ORAI channels. Our findings reveal that IP3R and PLC are potential new targets of mibefradil offering novel insights into the effects of this drug.
Keywords: ALC cells; Ca2+ signaling; Cav; HEK293 cells; LS8 cells; Mibefradil; PLC pathway.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Wiltshire HR, Sutton BM, Heeps G, Betty AM, Angus DW, Harris SR, et al. Metabolism of the calcium antagonist, mibefradil (POSICOR, Ro 40-5967). Part III. Comparative pharmacokinetics of mibefradil and its major metabolites in rat, marmoset, cynomolgus monkey and man. Xenobiotica. 1997;27(6):557–571. doi: 10.1080/004982597240343. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
