Identification of Cuticular and Web Lipids of the Spider Argiope bruennichi
- PMID: 35006525
- PMCID: PMC8934766
- DOI: 10.1007/s10886-021-01338-y
Identification of Cuticular and Web Lipids of the Spider Argiope bruennichi
Abstract
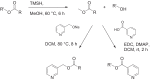
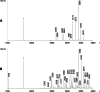
Emerging evidence shows that the cuticular and silk lipids of spiders are structurally more diverse than those of insects, although only a relatively low number of species have been investigated so far. As in insects, such lipids might play a role as signals in various contexts. The wasp spider Argiope bruennichi has probably the best investigated chemical communication system within spiders, including the known structure of the female sex pheromone. Recently we showed that kin-recognition in A. bruennichi could be mediated through the cuticular compounds consisting of hydrocarbons and, to a much larger proportion, of wax esters. By use of mass spectrometry and various derivatization methods, these were identified as esters of 2,4-dimethylalkanoic acids and 1-alkanols of varying chain lengths, such as tetradecyl 2,4-dimethylheptadecanoate. A representative enantioselective synthesis of this compound was performed which proved the identifications and allowed us to postulate that the natural enantiomer likely has the (2R,4R)-configuration. Chemical profiles of the silk and cuticular lipids of females were similar, while male cuticular profiles differed from those of females. Major components of the male cuticular lipids were tridecyl 2,4-dimethyl-C17-19 alkanoates, whereas those of females were slightly longer, comprising tridecyl 2,4-dimethyl-C19-21 alkanoates. In addition, minor female-specific 4-methylalkyl esters were detected.
Keywords: Branched fatty acids; GC/MS; Kin-recognition; Pheromones; Wax esters.
© 2022. The Author(s).
Conflict of interest statement
The authors have no financial or proprietary interests in any material discussed in this article.
Figures





Similar articles
-
Family-specific chemical profiles provide potential kin recognition cues in the sexually cannibalistic spider Argiope bruennichi.Biol Lett. 2021 Aug;17(8):20210260. doi: 10.1098/rsbl.2021.0260. Epub 2021 Aug 4. Biol Lett. 2021. PMID: 34343436 Free PMC article.
-
Spider sex pheromones: emission, reception, structures, and functions.Biol Rev Camb Philos Soc. 2007 Feb;82(1):27-48. doi: 10.1111/j.1469-185X.2006.00002.x. Biol Rev Camb Philos Soc. 2007. PMID: 17313523 Review.
-
Courtship pheromones in parasitic wasps: comparison of bioactive and inactive hydrocarbon profiles by multivariate statistical methods.J Chem Ecol. 2007 Apr;33(4):825-38. doi: 10.1007/s10886-007-9265-6. Epub 2007 Mar 1. J Chem Ecol. 2007. PMID: 17333370
-
Deciphering the signature of cuticular lipids with contact sex pheromone function in a parasitic wasp.J Exp Biol. 2012 Jul 15;215(Pt 14):2471-8. doi: 10.1242/jeb.071217. J Exp Biol. 2012. PMID: 22723487
-
Cuticular lipids of insects as potential biofungicides: methods of lipid composition analysis.Anal Bioanal Chem. 2011 Mar;399(9):3177-91. doi: 10.1007/s00216-010-4439-4. Epub 2010 Dec 12. Anal Bioanal Chem. 2011. PMID: 21153591 Review.
Cited by
-
Olfaction with legs-Spiders use wall-pore sensilla for pheromone detection.Proc Natl Acad Sci U S A. 2025 Jan 21;122(3):e2415468121. doi: 10.1073/pnas.2415468121. Epub 2025 Jan 6. Proc Natl Acad Sci U S A. 2025. PMID: 39761388 Free PMC article.
-
Host Plant Specificity in Web-Building Spiders.Insects. 2023 Feb 24;14(3):229. doi: 10.3390/insects14030229. Insects. 2023. PMID: 36975914 Free PMC article. Review.
-
Effect of Seasonal Variation on the Cuticular Chemical Composition of Atta laevigata (Smith 1858) (Hymenoptera: Formicidae).J Chem Ecol. 2025 Jan 31;51(1):15. doi: 10.1007/s10886-025-01559-5. J Chem Ecol. 2025. PMID: 39888559
-
The unique epicuticular chemistry of Collembola - A cross-species analysis.iScience. 2024 Jun 28;27(8):110416. doi: 10.1016/j.isci.2024.110416. eCollection 2024 Aug 16. iScience. 2024. PMID: 39139403 Free PMC article.
-
MACE - An Open Access Data Repository of Mass Spectra for Chemical Ecology.J Chem Ecol. 2022 Aug;48(7-8):589-597. doi: 10.1007/s10886-022-01364-4. J Chem Ecol. 2022. PMID: 35576088
References
-
- Aitchison J. The statistical analysis of compositional data. Caldwell: Blackburn Press; 2003.
-
- Blomquist GJ, Bagnères A-G. Insect Hydrocarbons: Biology, biochemistry, and chemical ecology. Cambridge: Cambridge University Press; 2010.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

