Phase II Trial of Inotuzumab Ozogamicin in Children and Adolescents With Relapsed or Refractory B-Cell Acute Lymphoblastic Leukemia: Children's Oncology Group Protocol AALL1621
- PMID: 35007127
- PMCID: PMC8937013
- DOI: 10.1200/JCO.21.01693
Phase II Trial of Inotuzumab Ozogamicin in Children and Adolescents With Relapsed or Refractory B-Cell Acute Lymphoblastic Leukemia: Children's Oncology Group Protocol AALL1621
Abstract
Purpose: Children's Oncology Group trial AALL1621 was conducted to prospectively determine the safety and efficacy of inotuzumab ozogamicin (InO) in pediatric and adolescent patients with relapsed or refractory (R/R) B-cell acute lymphoblastic leukemia (B-ALL).
Patients and methods: This single-arm phase II trial enrolled patients age 1-21 years with R/R CD22-positive B-ALL. In cycle 1, InO dosing was 0.8 mg/m2 intravenously on day 1 and 0.5 mg/m2 on days 8 and 15 of a 28-day cycle with response evaluation at day 28. Using a two-stage design, the trial was continuously monitored for dose-limiting toxicities and sinusoidal obstruction syndrome (SOS). CD22 expression was retrospectively evaluated by central flow cytometry.
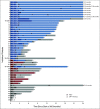
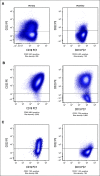
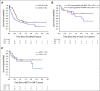
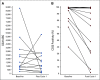
Results: Forty-eight patients were evaluable for response and toxicity; 19 had complete response (CR) and nine CR with incomplete count recovery (CRi) after cycle 1 (CR/CRi rate: 58.3%; two-sided 90% CI, 46.5 to 69.3). Twenty-seven of 28 patients with CR or CRi had minimal residual disease measured by flow cytometry; 18 (66.7%) had minimal residual disease < 0.01%. Seven of 28 patients (25%) with CR or CRi had delayed count recovery past day 42 in cycle 1. Three (6.3%) patients had grade 3 ALT elevation and one patient had grade 3 hyperbilirubinemia in cycle 1. Of 21 patients undergoing hematopoietic stem-cell transplantation after InO, 6 (28.6%) developed grade 3 SOS. Partial CD22 expression and lower CD22 site density were associated with lower likelihood of response to InO.
Conclusion: InO is effective and well tolerated in heavily pretreated children and adolescents with R/R CD22-positive B-ALL. SOS after hematopoietic stem-cell transplantation and prolonged cytopenias were notable. CD22 modulation was identified as a mechanism of resistance. Expanded study of InO combined with chemotherapy is underway.
Trial registration: ClinicalTrials.gov NCT02981628.
Conflict of interest statement
Figures
References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

