Analysis of the Clinical Pipeline of Treatments for Drug-Resistant Bacterial Infections: Despite Progress, More Action Is Needed
- PMID: 35007139
- PMCID: PMC8923189
- DOI: 10.1128/AAC.01991-21
Analysis of the Clinical Pipeline of Treatments for Drug-Resistant Bacterial Infections: Despite Progress, More Action Is Needed
Abstract
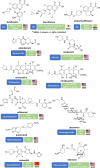
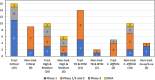
There is an urgent global need for new strategies and drugs to control and treat multidrug-resistant bacterial infections. In 2017, the World Health Organization (WHO) released a list of 12 antibiotic-resistant priority pathogens and began to critically analyze the antibacterial clinical pipeline. This review analyzes "traditional" and "nontraditional" antibacterial agents and modulators in clinical development current on 30 June 2021 with activity against the WHO priority pathogens mycobacteria and Clostridioides difficile. Since 2017, 12 new antibacterial drugs have been approved globally, but only vaborbactam belongs to a new antibacterial class. Also innovative is the cephalosporin derivative cefiderocol, which incorporates an iron-chelating siderophore that facilitates Gram-negative bacteria cell entry. Overall, there were 76 antibacterial agents in clinical development (45 traditional and 31 nontraditional), with 28 in phase 1, 32 in phase 2, 12 in phase 3, and 4 under regulatory evaluation. Forty-one out of 76 (54%) targeted WHO priority pathogens, 16 (21%) were against mycobacteria, 15 (20%) were against C. difficile, and 4 (5%) were nontraditional agents with broad-spectrum effects. Nineteen of the 76 antibacterial agents have new pharmacophores, and 4 of these have new modes of actions not previously exploited by marketed antibacterial drugs. Despite there being 76 antibacterial clinical candidates, this analysis indicated that there were still relatively few clinically differentiated antibacterial agents in late-stage clinical development, especially against critical-priority pathogens. We believe that future antibacterial research and development (R&D) should focus on the development of innovative and clinically differentiated candidates that have clear and feasible progression pathways to the market.
Keywords: Clostridioides difficile; WHO priority pathogens; antibacterial pipeline; antibiotic; clinical trials; mycobacteria; nontraditional; traditional; tuberculosis.
Conflict of interest statement
The authors declare a conflict of interest. The antibacterial pipeline data in this Minireview was built from the 2020 WHO Antibacterial Pipeline Report data using publicly available information as detailed in the manuscript’s Methodology section. Decisions on whether data would be included were voted upon by the full panel. If someone had declared a conflict of interest at the November 2020 meeting, then they were excluded from the discussion and voting. The following conflicts of interest were declared: Richard A. Alm works for CARB-X, Cesar A. Arias received support from MSD and Enstasis in the last four years, Roman Kozlov consulted for MSD and Pfizer between 2016 and 2020, Mical Paul consulted for Shionogi in 2021 and had a grant from Pfizer in 2020, Melvin Spigelman works for the TB Alliance, Guy E. Thwaites consulted for GSK over two years ago. John H. Rex is/has been Chief Medical Officer & Director, F2G, Ltd., Editor-in-Chief, AMR.Solutions, Operating Partner & Consultant, Advent Life Sciences, and has received grant support from Wellcome Trust; sits (or sat) on the scientific advisory boards of Bugworks Research, Inc., Basilea Pharmaceutica, Forge Therapeutics, Inc., Novo Holdings, Roche Pharma Research & Early Development, Sumitovant, and the AMR Action Fund (AMRAF); received consulting fees from Forge Therapeutics, Inc., Innocoll, Vedanta, Progenity, Nosopharm SA, Roivant Sciences, Shionogi Inc., GlaxoSmithKline, and Pfizer Pharmaceuticals. He is currently a shareholder in AstraZeneca Pharmaceuticals, F2G, Ltd, Advent Life Sciences, Zikani Therapeutics, and Bugworks Research, Inc. Laila Al-Sulaiman, Mark S. Butler, Peter Beyer, Lloyd Czaplewski, Prabhavathi Fernandes, Valeria Gigante, Stephan Harbarth, Christian Lienhardt, Norio Ohmagari, Sarah Paulin, and Hatim Sati declared no conflicts of interest. The opinions expressed in this Minireview do not necessarily reflect the opinion of any of the groups with which any of the authors works.
Figures



Comment in
-
Third-Generation Cephalosporin-Resistant Enterobacterales Are Critical Priority Pathogens, Too!Antimicrob Agents Chemother. 2022 Apr 19;66(4):e0021322. doi: 10.1128/aac.00213-22. Epub 2022 Mar 24. Antimicrob Agents Chemother. 2022. PMID: 35323013 Free PMC article. No abstract available.
-
Reply to Kaye and Belley, "Third-Generation Cephalosporin-Resistant Enterobacterales Are Critical Priority Pathogens, Too!".Antimicrob Agents Chemother. 2022 Apr 19;66(4):e0022322. doi: 10.1128/aac.00223-22. Epub 2022 Mar 24. Antimicrob Agents Chemother. 2022. PMID: 35323014 Free PMC article. No abstract available.
References
-
- Global AMR R&D Hub. 2021. Estimating global patient needs and market potential for priority health technologies addressing antimicrobial resistance. https://globalamrhub.org/our-work/studies/market-potential-and-priority-.... Global AMR R&D Hub, Berlin, Germany.
-
- World Health Organization. 2017. WHO publishes list of bacteria for which new antibiotics are urgently needed. https://www.who.int/news/item/27-02-2017-who-publishes-list-of-bacteria-.... WHO, Geneva, Switzerland.
-
- Tacconelli E, Carrara E, Savoldi A, Harbarth S, Mendelson M, Monnet DL, Pulcini C, Kahlmeter G, Kluytmans J, Carmeli Y, Ouellette M, Outterson K, Patel J, Cavaleri M, Cox EM, Houchens CR, Grayson ML, Hansen P, Singh N, Theuretzbacher U, Magrini N, WHO Pathogens Priority List Working Group. 2018. Discovery, research, and development of new antibiotics: the WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect Dis 18:318–327. 10.1016/S1473-3099(17)30753-3. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

