Naive T-Cell Depletion to Prevent Chronic Graft-Versus-Host Disease
- PMID: 35007144
- PMCID: PMC8987226
- DOI: 10.1200/JCO.21.01755
Naive T-Cell Depletion to Prevent Chronic Graft-Versus-Host Disease
Abstract
Purpose: Graft-versus-host disease (GVHD) causes morbidity and mortality following allogeneic hematopoietic cell transplantation. Naive T cells (TN) cause severe GVHD in murine models. We evaluated chronic GVHD (cGVHD) and other outcomes in three phase II clinical trials of TN-depletion of peripheral blood stem-cell (PBSC) grafts.
Methods: One hundred thirty-eight patients with acute leukemia received TN-depleted PBSC from HLA-matched related or unrelated donors following conditioning with high- or intermediate-dose total-body irradiation and chemotherapy. GVHD prophylaxis was with tacrolimus, with or without methotrexate or mycophenolate mofetil. Subjects received CD34-selected PBSC and a defined dose of memory T cells depleted of TN. Median follow-up was 4 years. The primary outcome of the analysis of cumulative data from the three trials was cGVHD.
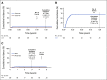
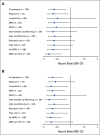
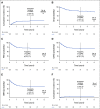
Results: cGVHD was very infrequent and mild (3-year cumulative incidence total, 7% [95% CI, 2 to 11]; moderate, 1% [95% CI, 0 to 2]; severe, 0%). Grade III and IV acute GVHD (aGVHD) occurred in 4% (95% CI, 1 to 8) and 0%, respectively. The cumulative incidence of grade II aGVHD, which was mostly stage 1 upper gastrointestinal GVHD, was 71% (95% CI, 64 to 79). Recipients of matched related donor and matched unrelated donor grafts had similar rates of grade III aGVHD (5% [95% CI, 0 to 9] and 4% [95% CI, 0 to 9]) and cGVHD (7% [95% CI, 2 to 13] and 6% [95% CI, 0 to 12]). Overall survival, cGVHD-free, relapse-free survival, relapse, and nonrelapse mortality were, respectively, 77% (95% CI, 71 to 85), 68% (95% CI, 61 to 76), 23% (95% CI, 16 to 30), and 8% (95% CI, 3 to 13) at 3 years.
Conclusion: Depletion of TN from PBSC allografts results in very low incidences of severe acute and any cGVHD, without apparent excess risks of relapse or nonrelapse mortality, distinguishing this novel graft engineering strategy from other hematopoietic cell transplantation approaches.
Trial registration: ClinicalTrials.gov NCT00914940 NCT01858740 NCT02220985.
Conflict of interest statement
Figures

Comment in
-
Naïve T-Cell Depletion to Prevent GVHD: Searching for a Better Mousetrap.J Clin Oncol. 2022 Apr 10;40(11):1139-1141. doi: 10.1200/JCO.22.00105. Epub 2022 Feb 25. J Clin Oncol. 2022. PMID: 35213234 No abstract available.
References
-
- Shlomchik WD. Graft-versus-host disease. Nat Rev Immunol. 2007;7:340–352. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

