Hyperinflammatory environment drives dysfunctional myeloid cell effector response to bacterial challenge in COVID-19
- PMID: 35007290
- PMCID: PMC8782468
- DOI: 10.1371/journal.ppat.1010176
Hyperinflammatory environment drives dysfunctional myeloid cell effector response to bacterial challenge in COVID-19
Abstract
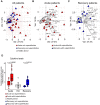
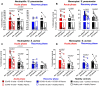
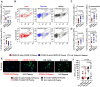
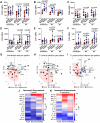
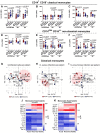
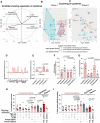
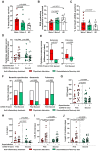
COVID-19 displays diverse disease severities and symptoms including acute systemic inflammation and hypercytokinemia, with subsequent dysregulation of immune cells. Bacterial superinfections in COVID-19 can further complicate the disease course and are associated with increased mortality. However, there is limited understanding of how SARS-CoV-2 pathogenesis and hypercytokinemia impede the innate immune function against bacterial superinfections. We assessed the influence of COVID-19 plasma hypercytokinemia on the functional responses of myeloid immune cells upon bacterial challenges from acute-phase COVID-19 patients and their corresponding recovery-phase. We show that a severe hypercytokinemia status in COVID-19 patients correlates with the development of bacterial superinfections. Neutrophils and monocytes derived from COVID-19 patients in their acute-phase showed an impaired intracellular microbicidal capacity upon bacterial challenges. The impaired microbicidal capacity was reflected by abrogated MPO and reduced NETs production in neutrophils along with reduced ROS production in both neutrophils and monocytes. Moreover, we observed a distinct pattern of cell surface receptor expression on both neutrophils and monocytes, in line with suppressed autocrine and paracrine cytokine signaling. This phenotype was characterized by a high expression of CD66b, CXCR4 and low expression of CXCR1, CXCR2 and CD15 in neutrophils and low expression of HLA-DR, CD86 and high expression of CD163 and CD11b in monocytes. Furthermore, the impaired antibacterial effector function was mediated by synergistic effect of the cytokines TNF-α, IFN-γ and IL-4. COVID-19 patients receiving dexamethasone showed a significant reduction of overall inflammatory markers in the plasma as well as exhibited an enhanced immune response towards bacterial challenge ex vivo. Finally, broad anti-inflammatory treatment was associated with a reduction in CRP, IL-6 levels as well as length of ICU stay and ventilation-days in critically ill COVID-19 patients. Our data provides insights into the transient functional dysregulation of myeloid immune cells against subsequent bacterial infections in COVID-19 patients and describe a beneficial role for the use of dexamethasone in these patients.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

