A Plausible Prebiotic One-Pot Synthesis of Orotate and Pyruvate Suggestive of Common Protometabolic Pathways
- PMID: 35007387
- PMCID: PMC8885966
- DOI: 10.1002/anie.202112572
A Plausible Prebiotic One-Pot Synthesis of Orotate and Pyruvate Suggestive of Common Protometabolic Pathways
Abstract
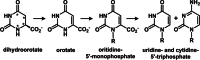
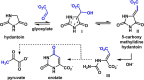
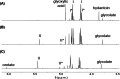
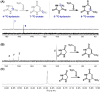
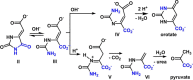
A reaction between two prebiotically plausible building blocks, hydantoin and glyoxylate, generates both the nucleobase orotate, a precursor of biological pyrimidines, and pyruvate, a core metabolite in the citric acid cycle and amino acid biosynthesis. The reaction proceeds in water to provide significant yields of the two widely divergent chemical motifs. Additionally, the reaction of thiohydantoin and glyoxylate produces thioorotate in high yield under neutral aqueous conditions. The use of an open-chain thiohydantoin derivative also enables the potential pre-positioning of a nucleosidic bond prior to the synthesis of an orotate nucleoside. The observation that diverse building blocks of modern metabolism can be produced in a single reaction pot, from common reactants under mild conditions, supports the plausibility of orthogonal chemistries operating at the origins of chemical evolution.
Keywords: Prebiotic Chemistry; Pyrimidine Synthesis; Pyruvate Synthesis.
© 2022 The Authors. Angewandte Chemie International Edition published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

