Allergy-induced systemic inflammation impairs tendon quality
- PMID: 35007819
- PMCID: PMC8749446
- DOI: 10.1016/j.ebiom.2021.103778
Allergy-induced systemic inflammation impairs tendon quality
Abstract
Background: Treatment of degenerating tendons still presents a major challenge, since the aetiology of tendinopathies remains poorly understood. Besides mechanical overuse, further known predisposing factors include rheumatoid arthritis, diabetes, obesity or smoking all of which combine with a systemic inflammation.
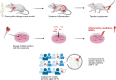
Methods: To determine whether the systemic inflammation accompanying these conditions contributes to the onset of tendinopathy, we studied the effect of a systemic inflammation induced by an allergic episode on tendon properties. To this end, we induced an allergic response in mice by exposing them to a timothy grass pollen allergen and subsequently analysed both their flexor and Achilles tendons. Additionally, we analysed data from a health survey comprising data from more than 10.000 persons for an association between the occurrence of an allergy and tendinopathy.
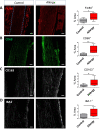
Findings: Biomechanical testing and histological analysis revealed that tendons from allergic mice not only showed a significant reduction of both elastic modulus and tensile stress, but also alterations of the tendon matrix. Moreover, treatment of 3D tendon-like constructs with sera from allergic mice resulted in a matrix-remodelling expression profile and the expression of macrophage-associated markers and matrix metalloproteinase 2 (MMP2) was increased in allergic Achilles tendons. Data from the human health study revealed that persons suffering from an allergy have an increased propensity to develop a tendinopathy.
Interpretation: Our study demonstrates that the presence of a systemic inflammation accompanying an allergic condition negatively impacts on tendon structure and function.
Funding: This study was financially supported by the Fund for the Advancement of Scientific Research at Paracelsus Medical University (PMU-FFF E-15/22/115-LEK), by the Land Salzburg, the Salzburger Landeskliniken (SALK, the Health Care Provider of the University Hospitals Landeskrankenhaus and Christian Doppler Klinik), the Paracelsus Medical University, Salzburg and by unrestricted grants from Bayer, AstraZeneca, Sanofi-Aventis, Boehringer-Ingelheim.
Keywords: Allergy; Systemic inflammation; Tendinopathy; Tendon.
Copyright © 2021 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of interest ET declares his interests as follows: Grants or contracts from any entity: Austrian Science Fund (FWF), Österreichische Nationalbank, European Union, GSK, Biogen, Eisai, Noartis, Red Bull, Bayer, UCB Consulting fees: UCB, Eisai, Bial, Medtronic, Everpharma, GSK, Liva-Nova, Newbridge, Novartis, Sanofi, Sandoz, Biogen, Takeda, Sunovion, GW Pharm, Marinus, Arvelle, Argenix, Epilog, Clexio Payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events: UCB, Eisai, Biogen, Novartis, Bial, Sunovion, Ever-Pharma, Liva-Nova, Sanofi, Hikma, Newbridge, Arvell, GW-Pharm, Sandoz Participation on a Data Safety Monitoring Board or Advisory Board: Marinus UCB
Figures






References
-
- Cho N.S., Yi J.W., Lee B.G., Rhee YG. Retear patterns after arthroscopic rotator cuff repair: single-row versus suture bridge technique. Am J Sport Med. 2010;38(4):664–671. - PubMed
-
- Choi S., Kim M.K., Kim G.M., Roh Y.H., Hwang I.K., Kang H. Factors associated with clinical and structural outcomes after arthroscopic rotator cuff repair with a suture bridge technique in medium, large, and massive tears. J Shoulder Elb Surg. 2014;23(11):1675–1681. - PubMed
-
- Millar N.L., Silbernagel K.G., Thorborg K., Kirwan P.D., Galatz L.M., Abrams G.D., et al. Tendinopathy. Nat Rev Dis Primers. 2021;7(1):1. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

