Implementation of large-scale laboratory-based detection of COVID-19 in the Veterans Health Administration, March 2020 - February 2021
- PMID: 35007825
- PMCID: PMC8665666
- DOI: 10.1016/j.diagmicrobio.2021.115617
Implementation of large-scale laboratory-based detection of COVID-19 in the Veterans Health Administration, March 2020 - February 2021
Abstract
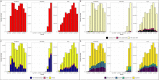
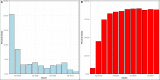
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) presented numerous operational challenges to healthcare delivery networks responsible for implementing large scale detection of Coronavirus Disease 2019 (COVID-19), the infection caused by SARS-CoV-2. We describe testing performance, review data quality metrics, and summarize experiences during the scale up of laboratory-based detection of COVID-19 in the Veterans Health Administration, the largest healthcare system in the United States. During March 2020 to February 2021, we observed rapid increase in testing volume, decreases in test turnaround time, improvements in testing of hospitalized persons, changes in test positivity, and varying utilization of different tests. Though performance metrics improved over time, surges challenged testing capacity and data quality remained suboptimal. Future planning efforts should focus on fortifying supply chains for consumables and equipment repair, optimizing distribution of testing workload across laboratories, and improving informatics to accurately monitor operations and intent for testing during a public health emergency.
Keywords: COVID-19; Laboratory performance; Surveillance.
Published by Elsevier Inc.
Figures

References
-
- Food and Drug Administration, Policy for Coronavirus Disease-2019 Tests During the Public Health Emergency (Revised). 2020.
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

