Ipidacrine (Axamon), A Reversible Cholinesterase Inhibitor, Improves Erectile Function in Male Rats With Diabetes Mellitus-Induced Erectile Dysfunction
- PMID: 35007992
- PMCID: PMC8847829
- DOI: 10.1016/j.esxm.2021.100477
Ipidacrine (Axamon), A Reversible Cholinesterase Inhibitor, Improves Erectile Function in Male Rats With Diabetes Mellitus-Induced Erectile Dysfunction
Abstract
Background: Management of diabetes mellitus-induced erectile dysfunction (DMED) is challenging because of its insufficient responses to phosphodiesterase type 5 inhibitors.
Aim: To compare the effects of ipidacrine, a reversible cholinesterase inhibitor, and sildenafil on DMED in a rat model of streptozotocin (STZ)-induced diabetes.
Methods: Erectile dysfunction (ED) caused by STZ-induced diabetes mellitus was modeled in adult male Wistar rats, which were randomized to 4 groups: untreated diabetic rats, sildenafil (5 mg/kg), ipidacrine (3.6 mg/kg) and ipidacrine (6.7 mg/kg). The test drug (ipidacrine), comparator (sildenafil) or control substance (1% starch solution) were administered orally for 5 days or 14 days. Erectile function was assessed by the change in the maximum intracavernous pressure (ICPmax) following cavernous nerve electrical stimulation. The mean arterial pressure (MAP) was recorded, and the ICPmax/MAP ratio was calculated. Sexual behavior, cholinesterase activity and blood testosterone level tests assessed.
Main outcome measure: The quantitative value of ICPmax/MAP 14 days after the start of administration of the test drug and the comparison drug.
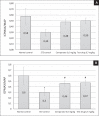
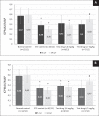
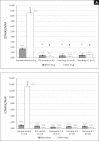
Results: Animals with STZ-induced diabetes mellitus showed a significant decrease in ICPmax and ICPmax/MAP ratio compared to the intact control group. When ipidacrine was administered to rats with DMED for 14 days, an increase in these indicators was noted. It was proved that ipidacrine at a dose of 6.7 mg/kg has noninferiority compared to sildenafil on the DMED model. Significant increase in ICPmax compared to STZ-control after electrostimulation of the cavernous nerve was recorded following administration of ipidacrine at a dose of 6.7 mg/kg (P < .05) and sildenafil at a dose 5 mg/kg (P < .05). Neither the test drug, nor the comparator were associated with increase in testosterone levels in blood; as well both drugs did not promote activation of sexual behavior.
Clinical implications: Ipidacrine may be considered as an effective therapy for DMED but needs to be verified in human investigations.
Strengths & limitations: The role of ipidacrine, was firstly demonstrated in rats with DMED. However, the results were obtained in animal experiments, and will be further tested in the study of receptor interactions and the determination of cellular targets.
Conclusion: This is the first study to show that administration of ipidacrine, the reversible cholinesterase inhibitor, improved erectile function in diabetic rats and these results may be beneficial in further studies using ipidacrine for treatment of DMED, particularly in non-responders to PDE5 inhibitors. Bykov V, Gushchina E, Morozov S, et al. Ipidacrine (Axamon), A Reversible Cholinesterase Inhibitor, Improves Erectile Function in Male Rats With Diabetes Mellitus-Induced Erectile Dysfunction. Sex Med 2022;10:100477.
Keywords: Erectile Dysfunction; Ipidacrine; Maximum Intracavernous Pressure.
Copyright © 2021 International Society for Sexual Medicine. Published by Elsevier Inc. All rights reserved.
Figures
References
-
- Castro RP, Hernández PC, Casilda RR, et al. Epidemiology of erectile dysfunction. Risk factors. Arch Esp Urol. 2010;8:637–639. - PubMed
-
- De Rose AF, Gallo F, Bini PM, et al. Epidemiology of sexual disorders in general medical practice: An Italian survey. Urologia. 2019;2:79–85. - PubMed
-
- Nicolosi A, Moreira ED, Jr, Shirai M, et al. Epidemiology of erectile dysfunction in four countries: Cross-national study of the prevalence and correlates of erectile dysfunction. Urology. 2003;1:201–206. - PubMed
-
- Fonseca V, Jawa A. Endothelial and erectile dysfunction, diabetes mellitus, and the metabolic syndrome: Common pathways and treatments? Am J Cardiol. 2005;12B:13M–18M. - PubMed
-
- Phé V, Rouprêt M. Erectile dysfunction and diabetes: A review of the current evidence- based medicine and a synthesis of the main available therapies. Diabetes Metab. 2012;1:1–13. - PubMed

