Eculizumab precision-dosing algorithm for thrombotic microangiopathy in children and young adults undergoing HSCT
- PMID: 35008105
- PMCID: PMC8905690
- DOI: 10.1182/bloodadvances.2021006523
Eculizumab precision-dosing algorithm for thrombotic microangiopathy in children and young adults undergoing HSCT
Abstract
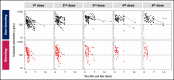
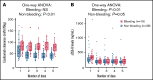
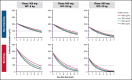
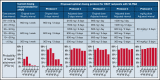
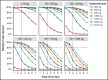
Transplant-associated thrombotic microangiopathy (TA-TMA) is a fatal posttransplant complication of hematopoietic stem cell transplantation. We recently reported that survival for TA-TMA has been improved by early intervention with eculizumab, a complement C5 inhibitor, guided by pharmacokinetic/pharmacodynamic (PK/PD) model-informed precision dosing. However, patients with gastrointestinal bleeding showed poor survival, even when treated with more frequent doses. Our objective was to develop separate models in bleeding and nonbleeding patients with TA-TMA and to propose precision dosing algorithms. Eculizumab PK/PD was analyzed in 19 bleeding and 38 nonbleeding patients (0.5-29.9 years of age). A complement activation biomarker (sC5b-9) and body weight were identified as significant determinants of eculizumab clearance regardless of bleeding. Eculizumab clearance after the first dose was higher in bleeding than in nonbleeding patients (83.8 vs 61.3 mL/h per 70 kg; P = .07). The high clearance was maintained over treatment doses in bleeding patients, whereas nonbleeding patients showed a time-dependent decrease in clearance. sC5b-9 levels were highest before the first dose and decreased over time, regardless of bleeding complications. A Monte Carlo Simulation analysis showed that the current dosing protocols recommended for atypical hemolytic uremic syndrome had <15% probability of attaining the target concentration of >100 μg/mL eculizumab in nonbleeding patients. We identified an intensified loading protocol to reach 80% target attainment. Our data clearly showed the need for individualized dosing for patients with significant bleeding and for ongoing dose adjustments to optimize outcomes. The developed models will be incorporated into a clinical decision guideline for precision dosing to improve outcomes in children and young adults with TA-TMA.
© 2022 by The American Society of Hematology. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Figures

References
-
- Jodele S. Complement in pathophysiology and treatment of transplant-associated thrombotic microangiopathies. Semin Hematol. 2018;55(3):159-166. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

