Identification of Neoadjuvant Chemotherapy Response in Muscle-Invasive Bladder Cancer by Fourier-Transform Infrared Micro-Imaging
- PMID: 35008184
- PMCID: PMC8750189
- DOI: 10.3390/cancers14010021
Identification of Neoadjuvant Chemotherapy Response in Muscle-Invasive Bladder Cancer by Fourier-Transform Infrared Micro-Imaging
Abstract
Background: Neoadjuvant chemotherapy (NAC) improves survival in responder patients. However, for non-responders, the treatment represents an ineffective exposure to chemotherapy and its potential adverse events. Predicting the response to treatment is a major issue in the therapeutic management of patients, particularly for patients with muscle-invasive bladder cancer.
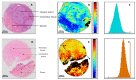
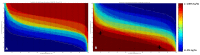
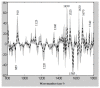
Methods: Tissue samples of trans-urethral resection of bladder tumor collected at the diagnosis time, were analyzed by mid-infrared imaging. A sequence of spectral data processing was implemented for automatic recognition of informative pixels and scoring each pixel according to a continuous scale (from 0 to 10) associated with the response to NAC. The ground truth status of the responder or non-responder was based on histopathological examination of the samples.
Results: Although the TMA spots of tumors appeared histologically homogeneous, the infrared approach highlighted spectral heterogeneity. Both the quantification of this heterogeneity and the scoring of the NAC response at the pixel level were used to construct sensitivity and specificity maps from which decision criteria can be extracted to classify cancerous samples.
Conclusions: This proof-of-concept appears as the first to evaluate the potential of the mid-infrared approach for the prediction of response to neoadjuvant chemotherapy in MIBC tissues.
Keywords: chemometric algorithms; mid-infrared imaging; muscle-invasive bladder cancer; neoadjuvant chemotherapy; predictive response to treatment.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Sonpavde G., Goldman B.H., Speights V.O., Lerner S.P., Wood D.P., Vogelzang N.J., Trump D.L., Natale R.B., Grossman H.B., Crawford E.D. Quality of pathologic response and surgery correlate with survival for patients with completely resected bladder cancer after neoadjuvant chemotherapy. Cancer. 2009;115:4104–4109. doi: 10.1002/cncr.24466. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

