Impact of Diabetes and Metformin Use on Enteropancreatic Neuroendocrine Tumors: Post Hoc Analysis of the CLARINET Study
- PMID: 35008233
- PMCID: PMC8750688
- DOI: 10.3390/cancers14010069
Impact of Diabetes and Metformin Use on Enteropancreatic Neuroendocrine Tumors: Post Hoc Analysis of the CLARINET Study
Abstract
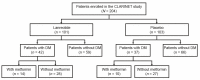
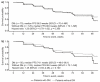
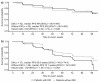
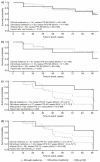
The prognostic role of diabetes mellitus (DM) in advanced enteropancreatic neuroendocrine tumors (NETs) is unclear. Progression free survival (PFS) was assessed in post-hoc analyses of the 96-week, phase III, double-blind, placebo-controlled CLARINET study of lanreotide 120 mg in patients with advanced non-functional enteropancreatic NETs with DM (with/without metformin) and without DM. Of 204 patients, there were 79 with DM (lanreotide, n = 42 {metformin, n = 14}; placebo, n = 37 {metformin, n = 10}) and 125 without DM (lanreotide, n = 59; placebo, n = 66). Median PFS was 96.0 and 98.0 weeks with and without DM, respectively (hazard ratio 1.20 {95% confidence interval 0.79 to 1.82}; p = 0.380). No difference in PFS was observed in lanreotide-treated patients with/without DM (p = 0.8476). In the placebo group, median PFS was numerically shorter with versus without DM (p = 0.052) and was significantly longer in patients with DM and metformin (85.7 weeks) versus without metformin (38.7 weeks; p = 0.009). Multivariable Cox analyses showed that DM at baseline was not associated with PFS (p = 0.079); lanreotide was significantly associated with lower disease progression risk (p = 0.017). Lanreotide efficacy was confirmed in patients with advanced enteropancreatic NETs, regardless of diabetic status; DM was not a negative prognostic factor. A potential antitumor effect of metformin was observed in patients receiving placebo.
Keywords: diabetes mellitus; lanreotide; progression-free survival.
Conflict of interest statement
S.P. received: grants and research funding from Ipsen and Pfizer; honoraria from Ipsen and Novartis; acted in a consulting/advisory role for Ipsen, Novartis, Pfizer and Advanced Accelerator Applications, Mylan, Merck. C.V. received research funding by Roche and acted in an advisory role for Novartis. M.D.M. received: research funding from Tesaro-GlaxoSmithKline; acted in a consulting/advisory role for Novartis, Pfizer, Eisai, Takeda, Janssen, Astellas, Roche, AstraZeneca. M.T. has nothing to disclose. N.P. and V.M. received honoraria from Ipsen. F.C. has nothing to disclose. J.C. has nothing to disclose. R.B. received: research funding and honoraria from Ipsen; acted in a consulting/advisory role for Ipsen. M.D.B. has nothing to disclose. M.M. has nothing to disclose. B.R. and X.-M.T.T. are employees of Ipsen. F.d.B. received: grants, research funding and honoraria from Ipsen; acted in a consulting/advisory role for Ipsen.
Figures

References
-
- Haugvik S.P., Hedenström P., Korsæth E., Valente R., Hayes A., Siuka D., Maisonneuve P., Gladhaug I.P., Lindkvist B., Capurso G. Diabetes, smoking, alcohol use, and family history of cancer as risk factors for pancreatic neuroendocrine tumors: A systematic review and meta-analysis. Neuroendocrinology. 2015;101:133–142. doi: 10.1159/000375164. - DOI - PubMed
-
- Santos A.P., Santos A.C., Castro C., Raposo L., Pereira S.S., Torres I., Henrique R., Cardoso H., Monteiro M.P. Visceral obesity and metabolic syndrome are associated with well-differentiated gastroenteropancreatic neuroendocrine tumors. Cancers. 2018;10:293. doi: 10.3390/cancers10090293. - DOI - PMC - PubMed
-
- Pusceddu S., Vernieri C., Di Maio M., Marconcini R., Spada F., Massironi S., Ibrahim T., Brizzi M., Campana D., Faggiano A., et al. Metformin use associates with longer progression-free survival of patients with diabetes and pancreatic neuroendocrine tumors receiving everolimus and/or somatostatin analogues. Gastroenterology. 2018;155:479–489.e7. doi: 10.1053/j.gastro.2018.04.010. - DOI - PubMed
LinkOut - more resources
Full Text Sources

