FDA-Approved Drugs for Hematological Malignancies-The Last Decade Review
- PMID: 35008250
- PMCID: PMC8750348
- DOI: 10.3390/cancers14010087
FDA-Approved Drugs for Hematological Malignancies-The Last Decade Review
Abstract
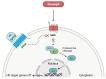
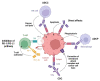
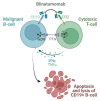
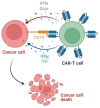
Hematological malignancies, also referred to as blood cancers, are a group of diseases involving abnormal cell growth and persisting in the blood, lymph nodes, or bone marrow. The development of new targeted therapies including small molecule inhibitors, monoclonal antibodies, bispecific T cell engagers, antibody-drug conjugates, recombinant immunotoxins, and, finally, Chimeric Antigen Receptor T (CAR-T) cells has improved the clinical outcomes for blood cancers. In this review, we summarized 52 drugs that were divided into small molecule and macromolecule agents, approved by the Food and Drug Administration (FDA) in the period between 2011 and 2021 for the treatment of hematological malignancies. Forty of them have also been approved by the European Medicines Agency (EMA). We analyzed the FDA-approved drugs by investigating both their structures and mechanisms of action. It should be emphasized that the number of targeted drugs was significantly higher (46 drugs) than chemotherapy agents (6 drugs). We highlight recent advances in the design of drugs that are used to treat hematological malignancies, which make them more effective and less toxic.
Keywords: EMA; FDA; hematological malignancies; macromolecule agents; small molecule agents.
Conflict of interest statement
The authors declare no conflict of interest.
Figures









Similar articles
-
FDA-Approved Small Molecule Compounds as Drugs for Solid Cancers from Early 2011 to the End of 2021.Molecules. 2022 Mar 31;27(7):2259. doi: 10.3390/molecules27072259. Molecules. 2022. PMID: 35408658 Free PMC article. Review.
-
Advanced targeted therapies in cancer: Drug nanocarriers, the future of chemotherapy.Eur J Pharm Biopharm. 2015 Jun;93:52-79. doi: 10.1016/j.ejpb.2015.03.018. Epub 2015 Mar 23. Eur J Pharm Biopharm. 2015. PMID: 25813885 Review.
-
Role of Immunotherapy in Targeting the Bone Marrow Microenvironment in Multiple Myeloma: An Evolving Therapeutic Strategy.Pharmacotherapy. 2017 Jan;37(1):129-143. doi: 10.1002/phar.1871. Epub 2017 Jan 6. Pharmacotherapy. 2017. PMID: 27870103 Review.
-
Approval of Cancer Drugs With Uncertain Therapeutic Value: A Comparison of Regulatory Decisions in Europe and the United States.Milbank Q. 2020 Dec;98(4):1219-1256. doi: 10.1111/1468-0009.12476. Epub 2020 Oct 6. Milbank Q. 2020. PMID: 33021339 Free PMC article.
-
Biological Therapy of Hematologic Malignancies: Toward a Chemotherapy- free Era.Curr Med Chem. 2019;26(6):1002-1018. doi: 10.2174/0929867324666171006144725. Curr Med Chem. 2019. PMID: 28990505 Review.
Cited by
-
Refining the relationship between gut microbiota and common hematologic malignancies: insights from a bidirectional Mendelian randomization study.Front Cell Infect Microbiol. 2024 Jun 14;14:1412035. doi: 10.3389/fcimb.2024.1412035. eCollection 2024. Front Cell Infect Microbiol. 2024. PMID: 38975324 Free PMC article.
-
Artificial Intelligence-Assisted Diagnostic Cytology and Genomic Testing for Hematologic Disorders.Cells. 2023 Jun 30;12(13):1755. doi: 10.3390/cells12131755. Cells. 2023. PMID: 37443789 Free PMC article. Review.
-
Crosstalk Between Autophagy and Oxidative Stress in Hematological Malignancies: Mechanisms, Implications, and Therapeutic Potential.Antioxidants (Basel). 2025 Feb 25;14(3):264. doi: 10.3390/antiox14030264. Antioxidants (Basel). 2025. PMID: 40227235 Free PMC article. Review.
-
Studies on the Complexation of Platinum(II) by Some 4-Nitroisoxazoles and Testing the Cytotoxic Activity of the Resulting Complexes.Molecules. 2023 Jan 28;28(3):1284. doi: 10.3390/molecules28031284. Molecules. 2023. PMID: 36770951 Free PMC article.
-
Metallic Nanoparticles: Their Potential Role in Breast Cancer Immunotherapy via Trained Immunity Provocation.Biomedicines. 2023 Apr 23;11(5):1245. doi: 10.3390/biomedicines11051245. Biomedicines. 2023. PMID: 37238916 Free PMC article. Review.
References
-
- Goodman L.S., Wintrobe M.M., Huang L., Huang J., Huang J., Xue H., Liang Z., Wu J., Chen C. Nitrogen mustard therapy; use of methyl-bis (beta-chloroethyl) amine hydrochloride and tris (beta-chloroethyl) amine hydrochloride for Hodgkin’s disease, lymphosarcoma, leukemia and certain allied and miscellaneous disorders. J. Am. Med. Assoc. 1946;132:126–132. doi: 10.1001/jama.1946.02870380008004. - DOI - PubMed
-
- Mustargen NDA #006695—Drugs@FDA: FDA-Approved Drugs. [(accessed on 23 August 2021)]; Available online: https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview....
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

