Physical Forces and Transient Nuclear Envelope Rupture during Metastasis: The Key for Success?
- PMID: 35008251
- PMCID: PMC8750110
- DOI: 10.3390/cancers14010083
Physical Forces and Transient Nuclear Envelope Rupture during Metastasis: The Key for Success?
Abstract
During metastasis, invading tumor cells and circulating tumor cells (CTC) face multiple mechanical challenges during migration through narrow pores and cell squeezing. However, little is known on the importance and consequences of mechanical stress for tumor progression and success in invading a new organ. Recently, several studies have shown that cell constriction can lead to nuclear envelope rupture (NER) during interphase. This loss of proper nuclear compartmentalization has a profound effect on the genome, being a key driver for the genome evolution needed for tumor progression. More than just being a source of genomic alterations, the transient nuclear envelope collapse can also support metastatic growth by several mechanisms involving the innate immune response cGAS/STING pathway. In this review we will describe the importance of the underestimated role of cellular squeezing in the progression of tumorigenesis. We will describe the complexity and difficulty for tumor cells to reach the metastatic site, detail the genomic aberration diversity due to NER, and highlight the importance of the activation of the innate immune pathway on cell survival. Cellular adaptation and nuclear deformation can be the key to the metastasis success in many unsuspected aspects.
Keywords: EMT; SASP; cGAS/STING; chromosomal instability; circulating tumor cells; mechanostress; metastasis; nuclear envelope rupture.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
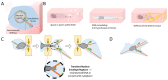
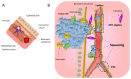

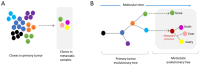

Similar articles
-
Nuclear Envelope Integrity in Health and Disease: Consequences on Genome Instability and Inflammation.Int J Mol Sci. 2021 Jul 6;22(14):7281. doi: 10.3390/ijms22147281. Int J Mol Sci. 2021. PMID: 34298904 Free PMC article. Review.
-
Nuclear rupture induced by capillary constriction forces promotes differential effects on metastatic and normal breast cells.Sci Rep. 2024 Jun 26;14(1):14793. doi: 10.1038/s41598-024-64733-x. Sci Rep. 2024. PMID: 38926422 Free PMC article.
-
The cGAS Paradox: Contrasting Roles for cGAS-STING Pathway in Chromosomal Instability.Cells. 2019 Oct 10;8(10):1228. doi: 10.3390/cells8101228. Cells. 2019. PMID: 31658669 Free PMC article. Review.
-
Consequences of a tight squeeze: Nuclear envelope rupture and repair.Nucleus. 2017 May 4;8(3):268-274. doi: 10.1080/19491034.2017.1292191. Epub 2017 Mar 13. Nucleus. 2017. PMID: 28287898 Free PMC article. Review.
-
Automated analysis of cell migration and nuclear envelope rupture in confined environments.PLoS One. 2018 Apr 12;13(4):e0195664. doi: 10.1371/journal.pone.0195664. eCollection 2018. PLoS One. 2018. PMID: 29649271 Free PMC article.
Cited by
-
Unlocking the therapeutic potential of the STING signaling pathway in anti-tumor treatment.Clin Exp Med. 2025 Aug 12;25(1):290. doi: 10.1007/s10238-025-01838-1. Clin Exp Med. 2025. PMID: 40794212 Free PMC article. Review.
-
Tissue mechanics in tumor heterogeneity and aggression.Trends Cancer. 2025 Aug;11(8):806-824. doi: 10.1016/j.trecan.2025.04.004. Epub 2025 Apr 29. Trends Cancer. 2025. PMID: 40307158 Review.
-
Bacterial Involvement in Progression and Metastasis of Colorectal Neoplasia.Cancers (Basel). 2022 Feb 17;14(4):1019. doi: 10.3390/cancers14041019. Cancers (Basel). 2022. PMID: 35205767 Free PMC article. Review.
-
At the nucleus of cancer: how the nuclear envelope controls tumor progression.MedComm (2020). 2025 Jan 24;6(2):e70073. doi: 10.1002/mco2.70073. eCollection 2025 Feb. MedComm (2020). 2025. PMID: 39866838 Free PMC article. Review.
-
Capillary constrictions prime cancer cell tumorigenicity through PIEZO1.Nat Commun. 2025 Sep 1;16(1):8160. doi: 10.1038/s41467-025-63374-6. Nat Commun. 2025. PMID: 40890143 Free PMC article.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

