Venetoclax for Children and Adolescents with Acute Lymphoblastic Leukemia and Lymphoblastic Lymphoma
- PMID: 35008312
- PMCID: PMC8750927
- DOI: 10.3390/cancers14010150
Venetoclax for Children and Adolescents with Acute Lymphoblastic Leukemia and Lymphoblastic Lymphoma
Abstract
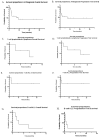
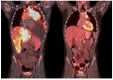
Venetoclax is approved for adult patients with chronic lymphocytic leukemia and acute myeloid leukemia. Expanding its use to the pediatric population is currently under investigation, but more robust data are needed. We retrospectively analyzed the safety and efficacy of venetoclax in children/AYA with ALL/LBL. We identified 18 patients (T-cell ALL, n = 7; T-cell LBL, n = 6; B-cell ALL, n = 5) aged 6-22 years. No new venetoclax safety signals were identified; the most common toxicity was myelosuppression. No deaths occurred within 30 days from the start of the therapy. A mean of 2.6 (range 0-8) prior lines of therapy were given. The mean duration of venetoclax was 4.06 months (range 0.2-24.67 months). Complete remission was achieved in 11 (61%) patients. Of the eight patients who remain alive, four are continuing on venetoclax combination therapy, and four proceeded to hematopoietic stem cell transplantation. Three patients who initially achieved CR, later relapsed, and are deceased. Nine patients are deceased, and one patient was lost to follow-up. Overall survival is 9.14 months (range 1.1-33.1), and progression-free survival is 7.34 months (range 0.2-33.1). This is the largest cohort of pediatric/AYA patients who received venetoclax for ALL/LBL. Our data support the consideration of venetoclax-based regimens in pediatric patients with R/R ALL/LBL and its investigation as upfront therapy for T-cell ALL/LBL.
Keywords: Bcl-2 inhibitor; acute lymphoblastic leukemia; early precursor T-cell; lymphoblastic lymphoma; venetoclax.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Clinical Experience With Venetoclax Combined With Chemotherapy for Relapsed or Refractory T-Cell Acute Lymphoblastic Leukemia.Clin Lymphoma Myeloma Leuk. 2020 Apr;20(4):212-218. doi: 10.1016/j.clml.2019.09.608. Epub 2019 Sep 30. Clin Lymphoma Myeloma Leuk. 2020. PMID: 32035785 Clinical Trial.
-
Venetoclax combination with Cladribine, idarubicin, Cytarabine for relapsed T-Cell acute lymphoblastic leukemia/lymphoblastic lymphoma treatment: A case report and literature review.Leuk Res Rep. 2025 Mar 14;23:100506. doi: 10.1016/j.lrr.2025.100506. eCollection 2025. Leuk Res Rep. 2025. PMID: 40206284 Free PMC article.
-
Poor outcome for children and adolescents with progressive disease or relapse of lymphoblastic lymphoma: a report from the berlin-frankfurt-muenster group.J Clin Oncol. 2009 Jul 10;27(20):3363-9. doi: 10.1200/JCO.2008.19.3367. Epub 2009 May 11. J Clin Oncol. 2009. PMID: 19433688
-
Venetoclax Combination Treatment of Acute Myeloid Leukemia in Adolescents and Young Adult Patients.J Clin Med. 2024 Apr 1;13(7):2046. doi: 10.3390/jcm13072046. J Clin Med. 2024. PMID: 38610812 Free PMC article. Review.
-
Cutaneous precursor B-cell lymphoblastic lymphoma in 2 adult patients: clinicopathologic and molecular cytogenetic studies with a review of the literature.Arch Dermatol. 2008 Sep;144(9):1155-62. doi: 10.1001/archderm.144.9.1155. Arch Dermatol. 2008. PMID: 18794461 Review.
Cited by
-
Current Status of Novel Agents for the Treatment of B Cell Malignancies: What's Coming Next?Cancers (Basel). 2022 Dec 7;14(24):6026. doi: 10.3390/cancers14246026. Cancers (Basel). 2022. PMID: 36551511 Free PMC article. Review.
-
Venetoclax in combination with chemotherapy as treatment for pediatric advanced hematologic malignancies.Pediatr Blood Cancer. 2023 Jun;70(6):e30335. doi: 10.1002/pbc.30335. Epub 2023 Apr 10. Pediatr Blood Cancer. 2023. PMID: 37036306 Free PMC article.
-
Remission rate, toxicity and pharmacokinetics of venetoclax-based induction regimens in untreated pediatric acute myeloid leukemia.NPJ Precis Oncol. 2024 Nov 2;8(1):248. doi: 10.1038/s41698-024-00740-5. NPJ Precis Oncol. 2024. PMID: 39488621 Free PMC article.
-
A phase 1/2 study of mini-hyper-CVD plus venetoclax in patients with relapsed/refractory acute lymphoblastic leukemia.Blood Adv. 2024 Feb 27;8(4):909-915. doi: 10.1182/bloodadvances.2023012231. Blood Adv. 2024. PMID: 38207208 Free PMC article. Clinical Trial.
-
Clinical experiences with venetoclax and other pro-apoptotic agents in lymphoid malignancies: lessons from monotherapy and chemotherapy combination.J Hematol Oncol. 2022 Jun 3;15(1):75. doi: 10.1186/s13045-022-01295-3. J Hematol Oncol. 2022. PMID: 35659041 Free PMC article. Review.
References
-
- Gaynon P.S., Angiolillo A.L., Carroll W.L., Nachman J.B., Trigg M.E., Sather H.N., Hunger S.P., Devidas M. Long-term results of the children’s cancer group studies for childhood acute lymphoblastic leukemia 1983–2002: A Children’s Oncology Group Report. Leukemia. 2010;24:285–297. doi: 10.1038/leu.2009.262. - DOI - PMC - PubMed
-
- Salzer W.L., Devidas M., Carroll W.L., Winick N., Pullen J., Hunger S.P., Camitta B.A. Long-term results of the pediatric oncology group studies for childhood acute lymphoblastic leukemia 1984–2001: A report from the children’s oncology group. Leukemia. 2010;24:355–370. doi: 10.1038/leu.2009.261. - DOI - PMC - PubMed
-
- Möricke A., Zimmermann M., Reiter A., Henze G., Schrauder A., Gadner H., Ludwig W.D., Ritter J., Harbott J., Mann G., et al. Long-term results of five consecutive trials in childhood acute lymphoblastic leukemia performed by the ALL-BFM study group from 1981 to 2000. Leukemia. 2010;24:265–284. doi: 10.1038/leu.2009.257. - DOI - PubMed
-
- Silverman L.B., Stevenson K.E., O’Brien J.E., Asselin B.L., Barr R.D., Clavell L., Cole P.D., Kelly K.M., Laverdiere C., Michon B., et al. Long-term results of Dana-Farber Cancer Institute ALL Consortium protocols for children with newly diagnosed acute lymphoblastic leukemia (1985–2000) Leukemia. 2010;24:320–334. doi: 10.1038/leu.2009.253. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Medical

