E-Cadherin-Deficient Epithelial Cells Are Sensitive to HDAC Inhibitors
- PMID: 35008338
- PMCID: PMC8749989
- DOI: 10.3390/cancers14010175
E-Cadherin-Deficient Epithelial Cells Are Sensitive to HDAC Inhibitors
Abstract
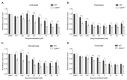
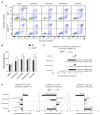
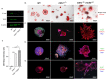
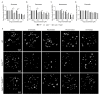
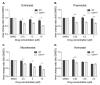
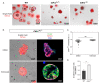
Inactivating germline mutations in the CDH1 gene (encoding the E-cadherin protein) are the genetic hallmark of hereditary diffuse gastric cancer (HDGC), and somatic CDH1 mutations are an early event in the development of sporadic diffuse gastric cancer (DGC) and lobular breast cancer (LBC). In this study, histone deacetylase (HDAC) inhibitors were tested for their ability to preferentially inhibit the growth of human cell lines (MCF10A and NCI-N87) and murine organoids lacking CDH1 expression. CDH1-/- breast and gastric cells were more sensitive to the pan-HDAC inhibitors entinostat, pracinostat, mocetinostat and vorinostat than wild-type cells, with an elevated growth inhibition that was, in part, attributable to increased apoptosis. CDH1-null cells were also sensitive to more class-specific HDAC inhibitors, but compared to the pan-inhibitors, these effects were less robust to genetic background. Increased sensitivity to entinostat was also observed in gastric organoids with both Cdh1 and Tp53 deletions. However, the deletion of Tp53 largely abrogated the sensitivity of the Cdh1-null organoids to pracinostat and mocetinostat. Finally, entinostat enhanced Cdh1 expression in heterozygous Cdh1+/- murine organoids. In conclusion, entinostat is a promising drug for the chemoprevention and/or treatment of HDGC and may also be beneficial for the treatment of sporadic CDH1-deficient cancers.
Keywords: CDH1; E-cadherin; HDAC inhibitors; HDGC; synthetic lethality.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Allosteric AKT Inhibitors Target Synthetic Lethal Vulnerabilities in E-Cadherin-Deficient Cells.Cancers (Basel). 2019 Sep 13;11(9):1359. doi: 10.3390/cancers11091359. Cancers (Basel). 2019. PMID: 31540244 Free PMC article.
-
E-Cadherin-Deficient Cells Are Sensitive to the Multikinase Inhibitor Dasatinib.Cancers (Basel). 2022 Mar 22;14(7):1609. doi: 10.3390/cancers14071609. Cancers (Basel). 2022. PMID: 35406381 Free PMC article.
-
Accuracy of Hereditary Diffuse Gastric Cancer Testing Criteria and Outcomes in Patients With a Germline Mutation in CDH1.Gastroenterology. 2015 Oct;149(4):897-906.e19. doi: 10.1053/j.gastro.2015.06.003. Epub 2015 Jun 11. Gastroenterology. 2015. PMID: 26072394
-
Cancer predisposition and germline CTNNA1 variants.Eur J Med Genet. 2021 Oct;64(10):104316. doi: 10.1016/j.ejmg.2021.104316. Epub 2021 Aug 21. Eur J Med Genet. 2021. PMID: 34425242
-
The role of the E-cadherin gene (CDH1) in diffuse gastric cancer susceptibility: from the laboratory to clinical practice.Ann Oncol. 2003 Dec;14(12):1705-13. doi: 10.1093/annonc/mdg486. Ann Oncol. 2003. PMID: 14630673 Review.
Cited by
-
Histone Deacetylase Functions in Gastric Cancer: Therapeutic Target?Cancers (Basel). 2022 Nov 7;14(21):5472. doi: 10.3390/cancers14215472. Cancers (Basel). 2022. PMID: 36358890 Free PMC article. Review.
-
Current advances in understanding the molecular profile of hereditary diffuse gastric cancer and its clinical implications.J Exp Clin Cancer Res. 2023 Mar 4;42(1):57. doi: 10.1186/s13046-023-02622-3. J Exp Clin Cancer Res. 2023. PMID: 36869400 Free PMC article. Review.
-
Hereditary diffuse gastric cancer: the evolution of a cancer syndrome.J R Soc N Z. 2025 Jun 16;55(6):2636-2651. doi: 10.1080/03036758.2025.2511007. eCollection 2025. J R Soc N Z. 2025. PMID: 40756848 Free PMC article. Review.
-
Non-targeted biopsies in hereditary diffuse gastric cancer: Necessary, but not enough.Transl Cancer Res. 2023 Jul 31;12(7):1883-1886. doi: 10.21037/tcr-23-541. Epub 2023 Jul 6. Transl Cancer Res. 2023. PMID: 37588744 Free PMC article. No abstract available.
-
Current advances and challenges in Managing Hereditary Diffuse Gastric Cancer (HDGC): a narrative review.Hered Cancer Clin Pract. 2024 Oct 8;22(1):21. doi: 10.1186/s13053-024-00293-5. Hered Cancer Clin Pract. 2024. PMID: 39379994 Free PMC article. Review.
References
-
- Blair V.R., McLeod M., Carneiro F., Coit D.G., D’Addario J.L., van Dieren J.M., Harris K.L., Hoogerbrugge N., Oliveira C., van der Post R.S., et al. Hereditary diffuse gastric cancer: Updated clinical practice guidelines. Lancet Oncol. 2020;21:e386–e397. doi: 10.1016/S1470-2045(20)30219-9. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

