A Shortcut from Metabolic-Associated Fatty Liver Disease (MAFLD) to Hepatocellular Carcinoma (HCC): c-MYC a Promising Target for Preventative Strategies and Individualized Therapy
- PMID: 35008356
- PMCID: PMC8750626
- DOI: 10.3390/cancers14010192
A Shortcut from Metabolic-Associated Fatty Liver Disease (MAFLD) to Hepatocellular Carcinoma (HCC): c-MYC a Promising Target for Preventative Strategies and Individualized Therapy
Abstract
Background: Metabolic-associated fatty liver disease (MAFLD) has risen as one of the leading etiologies for hepatocellular carcinoma (HCC). Oncogenes have been suggested to be responsible for the high risk of MAFLD-related HCC. We analyzed the impact of the proto-oncogene c-MYC in the development of human and murine MAFLD and MAFLD-associated HCC.
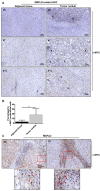
Methods: alb-myctg mice were studied at baseline conditions and after administration of Western diet (WD) in comparison to WT littermates. c-MYC expression was analyzed in biopsies of patients with MAFLD and MAFLD-associated HCC by immunohistochemistry.
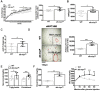
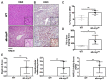
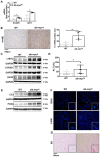
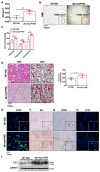
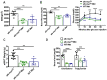
Results: Mild obesity, spontaneous hyperlipidaemia, glucose intolerance and insulin resistance were characteristic of 36-week-old alb-myctg mice. Middle-aged alb-myctg exhibited liver steatosis and increased triglyceride content. Liver injury and inflammation were associated with elevated ALT, an upregulation of ER-stress response and increased ROS production, collagen deposition and compensatory proliferation. At 52 weeks, 20% of transgenic mice developed HCC. WD feeding exacerbated metabolic abnormalities, steatohepatitis, fibrogenesis and tumor prevalence. Therapeutic use of metformin partly attenuated the spontaneous MAFLD phenotype of alb-myctg mice. Importantly, upregulation and nuclear localization of c-MYC were characteristic of patients with MAFLD and MAFLD-related HCC.
Conclusions: A novel function of c-MYC in MAFLD progression was identified opening new avenues for preventative strategies.
Keywords: c-myc; metabolic-associated fatty liver disease (MAFLD); metformin; oncogene; tumorigenesis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Estes C., Anstee Q.M., Arias-Loste M.T., Bantel H., Bellentani S., Caballeria J., Colombo M., Craxi A., Crespo J., Day C.P., et al. Modeling nafld disease burden in china, france, germany, italy, japan, spain, united kingdom, and united states for the period 2016–2030. J. Hepatol. 2018;69:896–904. doi: 10.1016/j.jhep.2018.05.036. - DOI - PubMed
Grants and funding
- SAF2016-78711, SAF2017-87919-R, PID2020-117827RB-IOO, PID2020-117941RB-IOO, PID2020-117116RB-I00/MINECO Retos
- S2017/BMD-3727/EXOHEP-CM
- Y2018/NMT-4949/NanoLiver-CM
- 2018/117/AMMF
- CA17112 and UCM-25/2019/COST Action
- HR17-00601/La Caixa Foundation Program
- SFB 1382-403224013/A02 and B07/German Research Foundation
- RYC-2014-15242 and RYC-2015-17438/Ramón y Cajal Researchers
- CT63/19./programa de Financiación de Universidad Complutense de Madrid - Banco Santander,
- PE-0451-2018/Proyectos Estratégicos-Fondos Feder
- PI16/01842, PI19/01404 & PI19/00589/Instituto de Salud Carlos III
LinkOut - more resources
Full Text Sources
Miscellaneous

