Natural and Synthetic Estrogens in Chronic Inflammation and Breast Cancer
- PMID: 35008370
- PMCID: PMC8744660
- DOI: 10.3390/cancers14010206
Natural and Synthetic Estrogens in Chronic Inflammation and Breast Cancer
Abstract
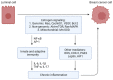
The oncogenic role of estrogen receptor (ER) signaling in breast cancer has long been established. Interaction of estrogen with estrogen receptor (ER) in the nucleus activates genomic pathways of estrogen signaling. In contrast, estrogen interaction with the cell membrane-bound G-protein-coupled estrogen receptor (GPER) activates the rapid receptor-mediated signaling transduction cascades. Aberrant estrogen signaling enhances mammary epithelial cell proliferation, survival, and angiogenesis, hence is an important step towards breast cancer initiation and progression. Meanwhile, a growing number of studies also provide evidence for estrogen's pro- or anti-inflammatory roles. As other articles in this issue cover classic ER and GPER signaling mediated by estrogen, this review will discuss the crucial mechanisms by which estrogen signaling influences chronic inflammation and how that is involved in breast cancer. Xenoestrogens acquired from plant diet or exposure to industrial products constantly interact with and alter innate estrogen signaling at various levels. As such, they can modulate chronic inflammation and breast cancer development. Natural xenoestrogens generally have anti-inflammatory properties, which is consistent with their chemoprotective role in breast cancer. In contrast, synthetic xenoestrogens are proinflammatory and carcinogenic compounds that can increase the risk of breast cancer. This article also highlights important xenoestrogens with a particular focus on their role in inflammation and breast cancer. Improved understanding of the complex relationship between estrogens, inflammation, and breast cancer will guide clinical research on agents that could advance breast cancer prevention and therapy.
Keywords: breast cancer; chronic inflammation; environmental estrogens; natural estrogens; synthetic estrogens; xenoestrogens.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

