Lipid Metabolism in Cancer: The Role of Acylglycerolphosphate Acyltransferases (AGPATs)
- PMID: 35008394
- PMCID: PMC8750616
- DOI: 10.3390/cancers14010228
Lipid Metabolism in Cancer: The Role of Acylglycerolphosphate Acyltransferases (AGPATs)
Abstract
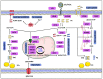
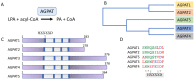
Altered lipid metabolism is an emerging hallmark of aggressive tumors, as rapidly proliferating cancer cells reprogram fatty acid (FA) uptake, synthesis, storage, and usage to meet their increased energy demands. Central to these adaptive changes, is the conversion of excess FA to neutral triacylglycerides (TAG) and their storage in lipid droplets (LDs). Acylglycerolphosphate acyltransferases (AGPATs), also known as lysophosphatidic acid acyltransferases (LPAATs), are a family of five enzymes that catalyze the conversion of lysophosphatidic acid (LPA) to phosphatidic acid (PA), the second step of the TAG biosynthesis pathway. PA, apart from its role as an intermediate in TAG synthesis, is also a precursor of glycerophospholipids and a cell signaling molecule. Although the different AGPAT isoforms catalyze the same reaction, they appear to have unique non-overlapping roles possibly determined by their distinct tissue expression and substrate specificity. This is best exemplified by the role of AGPAT2 in the development of type 1 congenital generalized lipodystrophy (CGL) and is also manifested by recent studies highlighting the involvement of AGPATs in the physiology and pathology of various tissues and organs. Importantly, AGPAT isoform expression has been shown to enhance proliferation and chemoresistance of cancer cells and correlates with increased risk of tumor development or aggressive phenotypes of several types of tumors.
Keywords: AGPAT; LPAAT; cancer; lipids; metabolism; phosphatidic acid.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
A novel lysophosphatidic acid acyltransferase enzyme (LPAAT4) with a possible role for incorporating docosahexaenoic acid into brain glycerophospholipids.Biochem Biophys Res Commun. 2014 Jan 10;443(2):718-24. doi: 10.1016/j.bbrc.2013.12.043. Epub 2013 Dec 12. Biochem Biophys Res Commun. 2014. PMID: 24333445
-
The lysophosphatidic acid acyltransferases (acylglycerophosphate acyltransferases) family: one reaction, five enzymes, many roles.Curr Opin Lipidol. 2018 Apr;29(2):110-115. doi: 10.1097/MOL.0000000000000492. Curr Opin Lipidol. 2018. PMID: 29373329 Review.
-
The Structure and Function of Acylglycerophosphate Acyltransferase 4/ Lysophosphatidic Acid Acyltransferase Delta (AGPAT4/LPAATδ).Front Cell Dev Biol. 2019 Aug 2;7:147. doi: 10.3389/fcell.2019.00147. eCollection 2019. Front Cell Dev Biol. 2019. PMID: 31428612 Free PMC article. Review.
-
AGPAT2 interaction with CDP-diacylglycerol synthases promotes the flux of fatty acids through the CDP-diacylglycerol pathway.Nat Commun. 2021 Nov 25;12(1):6877. doi: 10.1038/s41467-021-27279-4. Nat Commun. 2021. PMID: 34824276 Free PMC article.
-
Enzymatic activity of naturally occurring 1-acylglycerol-3-phosphate-O-acyltransferase 2 mutants associated with congenital generalized lipodystrophy.Biochem Biophys Res Commun. 2005 Feb 11;327(2):446-53. doi: 10.1016/j.bbrc.2004.12.024. Biochem Biophys Res Commun. 2005. PMID: 15629135
Cited by
-
Improving Recombinant Antibody Production Using FcBAR: An In Situ Approach to Detect and Amplify Protein-Protein Interactions.bioRxiv [Preprint]. 2025 Jun 17:2025.06.12.659199. doi: 10.1101/2025.06.12.659199. bioRxiv. 2025. Update in: Metab Eng. 2025 Jul 23;92:174-184. doi: 10.1016/j.ymben.2025.07.006. PMID: 40667234 Free PMC article. Updated. Preprint.
-
Lysophosphatidic acid triggers inflammation in the liver and white adipose tissue in rat models of 1-acyl-sn-glycerol-3-phosphate acyltransferase 2 deficiency and overnutrition.Proc Natl Acad Sci U S A. 2023 Dec 26;120(52):e2312666120. doi: 10.1073/pnas.2312666120. Epub 2023 Dec 21. Proc Natl Acad Sci U S A. 2023. PMID: 38127985 Free PMC article.
-
Improving recombinant antibody production using FcBAR: An in situ approach to detect and amplify protein-protein interactions.Metab Eng. 2025 Nov;92:174-184. doi: 10.1016/j.ymben.2025.07.006. Epub 2025 Jul 23. Metab Eng. 2025. PMID: 40712833 Free PMC article.
-
Metabolic gatekeepers: harnessing tumor-derived metabolites to optimize T cell-based immunotherapy efficacy in the tumor microenvironment.Cell Death Dis. 2024 Oct 26;15(10):775. doi: 10.1038/s41419-024-07122-6. Cell Death Dis. 2024. PMID: 39461979 Free PMC article. Review.
-
Unveiling the MUFA-Cancer Connection: Insights from Endogenous and Exogenous Perspectives.Int J Mol Sci. 2023 Jun 8;24(12):9921. doi: 10.3390/ijms24129921. Int J Mol Sci. 2023. PMID: 37373069 Free PMC article. Review.
References
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

