Harnessing Antitumor CD4+ T Cells for Cancer Immunotherapy
- PMID: 35008422
- PMCID: PMC8750687
- DOI: 10.3390/cancers14010260
Harnessing Antitumor CD4+ T Cells for Cancer Immunotherapy
Abstract
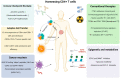
Over the past decades, CD4+ T cells have been considered as a supporting actor in the fields of cancer immunotherapy. Until recently, accumulating evidence has demonstrated the critical role of CD4+ T cells during antitumor immunity. CD4+ T cells can either suppress or promote the antitumor cytotoxic CD8+ T cell responses, either in secondary lymphoid organs or in the tumor. In this review, we provide an overview of the multifaceted role of different CD4+ T cell subsets in cancer immune response and their contribution during cancer therapies. Specifically, we focus on the latest progress regarding the impact of CD4+ T cell modulation on immunotherapies and other cancer therapies and discuss the prospect for harnessing CD4+ T cells to control tumor progression and prevent recurrence in patients.
Keywords: CD4+ T cells; adoptive cell transfer; cancer immunotherapy; cancer vaccine; immune checkpoint inhibitors.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
The outstanding antitumor capacity of CD4+ T helper lymphocytes.Biochim Biophys Acta Rev Cancer. 2020 Dec;1874(2):188439. doi: 10.1016/j.bbcan.2020.188439. Epub 2020 Sep 24. Biochim Biophys Acta Rev Cancer. 2020. PMID: 32980465 Review.
-
Revisiting the role of CD4+ T cells in cancer immunotherapy-new insights into old paradigms.Cancer Gene Ther. 2021 Feb;28(1-2):5-17. doi: 10.1038/s41417-020-0183-x. Epub 2020 May 27. Cancer Gene Ther. 2021. PMID: 32457487 Free PMC article. Review.
-
Are Conventional Type 1 Dendritic Cells Critical for Protective Antitumor Immunity and How?Front Immunol. 2019 Feb 12;10:9. doi: 10.3389/fimmu.2019.00009. eCollection 2019. Front Immunol. 2019. PMID: 30809220 Free PMC article. Review.
-
The role of CD4+ T cells in tumor and chronic viral immune responses.MedComm (2020). 2023 Oct 10;4(5):e390. doi: 10.1002/mco2.390. eCollection 2023 Oct. MedComm (2020). 2023. PMID: 37829505 Free PMC article. Review.
-
CD4+ T cell exhaustion leads to adoptive transfer therapy failure which can be prevented by immune checkpoint blockade.Am J Cancer Res. 2020 Dec 1;10(12):4234-4250. eCollection 2020. Am J Cancer Res. 2020. PMID: 33414997 Free PMC article.
Cited by
-
WW domain binding protein 2 (WBP2) as an oncogene in breast cancer: mechanisms and therapeutic prospects-a narrative review.Gland Surg. 2022 Dec;11(12):1984-2002. doi: 10.21037/gs-22-716. Gland Surg. 2022. PMID: 36654949 Free PMC article. Review.
-
Necroptosis-Related Genes Associated With Immune Activity and Prognosis of Colorectal Cancer.Front Genet. 2022 Jun 16;13:909245. doi: 10.3389/fgene.2022.909245. eCollection 2022. Front Genet. 2022. PMID: 35783272 Free PMC article.
-
Establishment of a high-fidelity patient-derived xenograft model for cervical cancer enables the evaluation of patient's response to conventional and novel therapies.J Transl Med. 2023 Sep 9;21(1):611. doi: 10.1186/s12967-023-04444-5. J Transl Med. 2023. PMID: 37689699 Free PMC article.
-
Development and therapeutic manipulation of the head and neck cancer tumor environment to improve clinical outcomes.Front Oral Health. 2022 Jul 22;3:902160. doi: 10.3389/froh.2022.902160. eCollection 2022. Front Oral Health. 2022. PMID: 35937775 Free PMC article. Review.
-
Tumor-Specific CD4+ T Cells Restrain Established Metastatic Melanoma by Developing Into Cytotoxic CD4- T Cells.Front Immunol. 2022 Jun 16;13:875718. doi: 10.3389/fimmu.2022.875718. eCollection 2022. Front Immunol. 2022. PMID: 35784297 Free PMC article.
References
-
- Sharma P., Siddiqui B.A., Anandhan S., Yadav S.S., Subudhi S.K., Gao J., Goswami S., Allison J.P. The Next Decade of Immune Checkpoint Therapy. Cancer Discov. 2021;11:838–857. doi: 10.1158/2159-8290.CD-20-1680. - DOI - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

