High-Dose Benzodiazepines Positively Modulate GABAA Receptors via a Flumazenil-Insensitive Mechanism
- PMID: 35008465
- PMCID: PMC8744940
- DOI: 10.3390/ijms23010042
High-Dose Benzodiazepines Positively Modulate GABAA Receptors via a Flumazenil-Insensitive Mechanism
Abstract
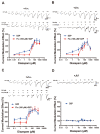
Benzodiazepines (BZDs) produce versatile pharmacological actions through positive modulation of GABAA receptors (GABAARs). A previous study has demonstrated that high concentrations of diazepam potentiate GABA currents on the α1β2γ2 and α1β2 GABAARs in a flumazenil-insensitive manner. In this study, the high-concentration effects of BZDs and their sensitivity to flumazenil were determined on synaptic (α1β2γ2, α2β2γ2, α5β2γ2) and extra-synaptic (α4β2δ) GABAARs using the voltage-clamp electrophysiology technique. The in vivo evaluation of flumazenil-insensitive BZD effects was conducted in mice via the loss of righting reflex (LORR) test. Diazepam induced biphasic potentiation on the α1β2γ2, α2β2γ2 and α5β2γ2 GABAARs, but did not affect the α4β2δ receptor. In contrast to the nanomolar component of potentiation, the second potentiation elicited by micromolar diazepam was insensitive to flumazenil. Midazolam, clonazepam, and lorazepam at 200 µM exhibited similar flumazenil-insensitive effects on the α1β2γ2, α2β2γ2 and α5β2γ2 receptors, whereas the potentiation induced by 200 µM zolpidem or triazolam was abolished by flumazenil. Both the GABAAR antagonist pentylenetetrazol and Fa173, a proposed transmembrane site antagonist, abolished the potentiation induced by 200 µM diazepam. Consistent with the in vitro results, flumazenil antagonized the zolpidem-induced LORR, but not that induced by diazepam or midazolam. Pentylenetetrazol and Fa173 antagonized the diazepam-induced LORR. These findings support the existence of non-classical BZD binding sites on certain GABAAR subtypes and indicate that the flumazenil-insensitive effects depend on the chemical structures of BZD ligands.
Keywords: GABAA receptors; benzodiazepine; loss of righting reflex; non-classical binding site; voltage-clamp electrophysiology.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures




Similar articles
-
Flumazenil-insensitive benzodiazepine binding sites in GABAA receptors contribute to benzodiazepine-induced immobility in zebrafish larvae.Life Sci. 2019 Dec 15;239:117033. doi: 10.1016/j.lfs.2019.117033. Epub 2019 Nov 4. Life Sci. 2019. PMID: 31697950
-
Benzodiazepines act on GABAA receptors via two distinct and separable mechanisms.Nat Neurosci. 2000 Dec;3(12):1274-81. doi: 10.1038/81800. Nat Neurosci. 2000. PMID: 11100148
-
Flumazenil-Insensitive Benzodiazepine Effects in Recombinant αβ and Neuronal GABAA Receptors.Brain Sci. 2020 Mar 5;10(3):150. doi: 10.3390/brainsci10030150. Brain Sci. 2020. PMID: 32150806 Free PMC article.
-
Midazolam and other benzodiazepines.Handb Exp Pharmacol. 2008;(182):335-60. doi: 10.1007/978-3-540-74806-9_16. Handb Exp Pharmacol. 2008. PMID: 18175099 Review.
-
Clerodane Diterpenes from Casearia corymbosa as Allosteric GABAA Receptor Modulators.J Nat Prod. 2022 May 27;85(5):1201-1210. doi: 10.1021/acs.jnatprod.1c00840. Epub 2022 Apr 27. J Nat Prod. 2022. PMID: 35475609 Free PMC article. Review.
References
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources

