Elevation of Hyaluronan Synthase by Magnesium Supplementation Mediated through the Activation of GSK3 and CREB in Human Keratinocyte-Derived HaCaT Cells
- PMID: 35008494
- PMCID: PMC8744730
- DOI: 10.3390/ijms23010071
Elevation of Hyaluronan Synthase by Magnesium Supplementation Mediated through the Activation of GSK3 and CREB in Human Keratinocyte-Derived HaCaT Cells
Abstract
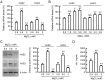
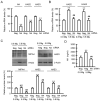
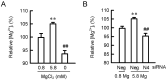
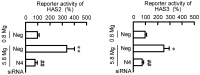
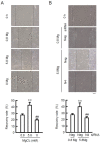
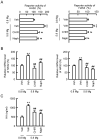
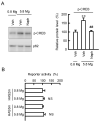
Skin barrier damage is present in the patients with hereditary disorders of the magnesium channel, but the molecular mechanism has not been fully understood. We found that the expressions of hyaluronan synthase (HAS), HAS2 and HAS3 are influenced by MgCl2 concentration in human keratinocyte-derived HaCaT cells. The exposure of cells to a high concentration (5.8 mM) of MgCl2 induced the elevation of HAS2/3 expression, which was inhibited by mRNA knockdown of nonimprinted in Prader-Willi/Angelman syndrome-like domain containing 4 (NIPAL4). Similarly, the content of hyaluronic acid (HA) was changed according to MgCl2 concentration and the expression of NIPAL4. The MgCl2 supplementation increased the reporter activities of HAS2/3, which were inhibited by NIPAL4 knockdown, indicating that the expressions of HAS2/3 are up-regulated at the transcriptional level. The reporter activities and mRNA levels of HAS2/3, and the production of HA were inhibited by CHIR-99021, a glycogen synthase kinase-3 (GSK3) inhibitor, and naphthol AS-E, a cyclic AMP-response element binding protein (CREB) inhibitor. Furthermore, the mutation in putative CREB-binding sites of promoter region in HAS2/3 genes inhibited the MgCl2 supplementation-induced elevation of promoter activity. Our results indicate that the expressions of HAS2/3 are up-regulated by MgCl2 supplementation in HaCaT cells mediated through the activation of GSK3 and CREB. Magnesium may play a pivotal role in maintaining the skin barrier function and magnesium supplementation may be useful to enhance moisturization and wound repair in the skin.
Keywords: hyaluronan synthase; hyaluronic acid; magnesium.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Oji V., Tadini G., Akiyama M., Bardon C.B., Bodemer C., Bourrat E., Coudiere P., DiGiovanna J.J., Elias P., Fischer J., et al. Revised nomenclature and classification of inherited ichthyoses: Results of the First Ichthyosis Consensus Conference in Soreze 2009. J. Am. Acad. Dermatol. 2010;63:607–641. doi: 10.1016/j.jaad.2009.11.020. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

