Insights into the Mechanisms of Action of MDA-7/IL-24: A Ubiquitous Cancer-Suppressing Protein
- PMID: 35008495
- PMCID: PMC8744595
- DOI: 10.3390/ijms23010072
Insights into the Mechanisms of Action of MDA-7/IL-24: A Ubiquitous Cancer-Suppressing Protein
Abstract
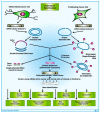
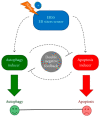
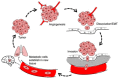
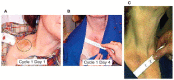
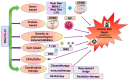
Melanoma differentiation associated gene-7/interleukin-24 (MDA-7/IL-24), a secreted protein of the IL-10 family, was first identified more than two decades ago as a novel gene differentially expressed in terminally differentiating human metastatic melanoma cells. MDA-7/IL-24 functions as a potent tumor suppressor exerting a diverse array of functions including the inhibition of tumor growth, invasion, angiogenesis, and metastasis, and induction of potent "bystander" antitumor activity and synergy with conventional cancer therapeutics. MDA-7/IL-24 induces cancer-specific cell death through apoptosis or toxic autophagy, which was initially established in vitro and in preclinical animal models in vivo and later in a Phase I clinical trial in patients with advanced cancers. This review summarizes the history and our current understanding of the molecular/biological mechanisms of MDA-7/IL-24 action rendering it a potent cancer suppressor.
Keywords: MDA-7/IL-24; apoptosis; bystander antitumor activity; combinatorial therapy; cytokine.
Conflict of interest statement
P.B.F. and W.K.C. are scientific co-founders and have equity in InterLeukin Combinatorial Therapies, Inc. (ILCT). VCU also has equity in ILCT. L.E. is the PI of a sponsored research agreement with ILCT, which is being managed by VCU.
Figures



References
-
- Jiang H., Fisher P.B. Use of a Sensitive and Efficient Subtraction Hybridization Protocol for the Identification of Genes Differentially Regulated during the Induction of Differentiation in Human Melanoma Cells. Mol. Cell. Differ. 1993;1:285–299.
-
- Jiang H., Lin J.J., Su Z.Z., Goldstein N.I., Fisher P.B. Subtraction Hybridization Identifies a Novel Melanoma Differentiation Associated Gene, Mda-7, Modulated during Human Melanoma Differentiation, Growth and Progression. Oncogene. 1995;11:2477–2486. - PubMed
-
- Su Z.Z., Madireddi M.T., Lin J.J., Young C.S., Kitada S., Reed J.C., Goldstein N.I., Fisher P.B. The cancer growth suppressor gene mda-7 selectively induces apoptosis in human breast cancer cells and inhibits tumor growth in nude mice. Proc. Natl. Acad. Sci. USA. 1998;95:14400–14405. doi: 10.1073/pnas.95.24.14400. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

