Targeting MAPK/NF-κB Pathways in Anti-Inflammatory Potential of Rutaecarpine: Impact on Src/FAK-Mediated Macrophage Migration
- PMID: 35008520
- PMCID: PMC8745017
- DOI: 10.3390/ijms23010092
Targeting MAPK/NF-κB Pathways in Anti-Inflammatory Potential of Rutaecarpine: Impact on Src/FAK-Mediated Macrophage Migration
Abstract
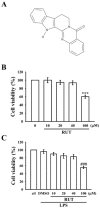
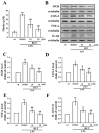
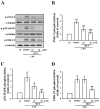
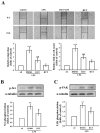
Studies have discovered that different extracts of Evodia rutaecarpa and its phytochemicals show a variety of biological activities associated with inflammation. Although rutaecarpine, an alkaloid isolated from the unripe fruit of E. rutaecarpa, has been exposed to have anti-inflammatory properties, the mechanism of action has not been well studied. Thus, this study investigated the molecular mechanisms of rutaecarpine (RUT) in lipopolysaccharide (LPS)-induced RAW 264.7 macrophages. RUT reserved the production of nitric oxide (NO) and the expression of inducible nitric oxide synthase (iNOS), cyclooxygenase-2 (COX-2), tumor necrosis factor (TNF-α), and interleukin (IL)-1β in the LPS-induced macrophages. RUT showed an inhibitory effect on the mitogen-activated protein kinases (MAPKs), and it also inhibited nuclear transcription factor kappa-B (NF-κB) by hindering IκBα and NF-κB p65 phosphorylation and p65 nuclear translocation. The phospho-PI3K and Akt was concentration-dependently suppressed by RUT. However, RUT not only suggestively reduced the migratory ability of macrophages and their numbers induced by LPS but also inhibited the phospho-Src, and FAK. Taken together, these results indicate that RUT participates a vital role in the inhibition of LPS-induced inflammatory processes in RAW 264.7 macrophages and that the mechanisms involve PI3K/Akt and MAPK-mediated downregulation of NF-κB signaling pathways. Notably, reducing the migration and number of cells induced by LPS via inhibiting of Src/FAK pathway was also included to the anti-inflammatory mechanism of RUT. Therefore, RUT may have potential benefits as a therapeutic agent against chronic inflammatory diseases.
Keywords: MAPK; NF-κB; PI3K/Akt; Src/FAK; anti-inflammation; cell migration; molecular mechanism; rutaecarpine.
Conflict of interest statement
The authors declare no conflict of interests.
Figures



Similar articles
-
Anti-Inflammatory Mechanism of An Alkaloid Rutaecarpine in LTA-Stimulated RAW 264.7 Cells: Pivotal Role on NF-κB and ERK/p38 Signaling Molecules.Int J Mol Sci. 2022 May 24;23(11):5889. doi: 10.3390/ijms23115889. Int J Mol Sci. 2022. PMID: 35682568 Free PMC article.
-
Eugenolol and glyceryl-isoeugenol suppress LPS-induced iNOS expression by down-regulating NF-kappaB AND AP-1 through inhibition of MAPKS and AKT/IkappaBalpha signaling pathways in macrophages.Int J Immunopathol Pharmacol. 2011 Apr-Jun;24(2):345-56. doi: 10.1177/039463201102400208. Int J Immunopathol Pharmacol. 2011. PMID: 21658309
-
Rutaecarpine Attenuates Monosodium Urate Crystal-Induced Gouty Inflammation via Inhibition of TNFR-MAPK/NF-κB and NLRP3 Inflammasome Signaling Pathways.Chin J Integr Med. 2025 Jul;31(7):590-599. doi: 10.1007/s11655-025-4204-3. Epub 2025 May 8. Chin J Integr Med. 2025. PMID: 40338445
-
Crosstalk between phytochemicals and inflammatory signaling pathways.Inflammopharmacology. 2023 Jun;31(3):1117-1147. doi: 10.1007/s10787-023-01206-z. Epub 2023 Apr 6. Inflammopharmacology. 2023. PMID: 37022574 Review.
-
Anti-inflammatory Effects of Compounds Extracted from Marine Sponge s: A Systematic Review.Antiinflamm Antiallergy Agents Med Chem. 2023;22(3):164-197. doi: 10.2174/0118715230272152231106094727. Antiinflamm Antiallergy Agents Med Chem. 2023. PMID: 38038014
Cited by
-
Anti-Inflammatory, Antibacterial, Anti-Biofilm, and Anti-Quorum Sensing Activities of the Diterpenes Isolated from Clinopodium bolivianum.Pharmaceutics. 2024 Aug 20;16(8):1094. doi: 10.3390/pharmaceutics16081094. Pharmaceutics. 2024. PMID: 39204439 Free PMC article.
-
Dietary Glyceryl Monolaurate Supplementation During Pregnancy Enhances Fetal Intrauterine Development and Antioxidant Capacity in Sows via Microbiota Modulation.Antioxidants (Basel). 2025 Jun 25;14(7):783. doi: 10.3390/antiox14070783. Antioxidants (Basel). 2025. PMID: 40722887 Free PMC article.
-
Biotechnological potential of Cannabis sativa adventitious roots for producing immunomodulatory and anti-inflammatory bioactive compounds.Sci Rep. 2025 Aug 22;15(1):30904. doi: 10.1038/s41598-025-16130-1. Sci Rep. 2025. PMID: 40847038 Free PMC article.
-
Stiffness and surface topology of silicone implants competitively mediate inflammatory responses of macrophages and foreign body response.Mater Today Bio. 2024 Oct 18;29:101304. doi: 10.1016/j.mtbio.2024.101304. eCollection 2024 Dec. Mater Today Bio. 2024. PMID: 39498150 Free PMC article.
-
Curcumin Ameliorates Particulate Matter-Induced Pulmonary Injury through Bimodal Regulation of Macrophage Inflammation via NF-κB and Nrf2.Int J Mol Sci. 2023 Jan 17;24(3):1858. doi: 10.3390/ijms24031858. Int J Mol Sci. 2023. PMID: 36768180 Free PMC article.
References
-
- Renda G., Tacconelli S., Capone M., Sacchetta D., Santarelli F., Sciulli M.G., Zimarino M., Grana M., D’Amelio E., Zurro M., et al. Celecoxib, Ibuprofen, and the Antiplatelet Effect of Aspirin in Patients with Osteoarthritis and Ischemic Heart Disease. Clin. Pharmacol. Ther. 2006;80:264–274. doi: 10.1016/j.clpt.2006.05.004. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

