Human iPSC-Derived Astrocytes: A Powerful Tool to Study Primary Astrocyte Dysfunction in the Pathogenesis of Rare Leukodystrophies
- PMID: 35008700
- PMCID: PMC8745131
- DOI: 10.3390/ijms23010274
Human iPSC-Derived Astrocytes: A Powerful Tool to Study Primary Astrocyte Dysfunction in the Pathogenesis of Rare Leukodystrophies
Abstract
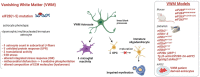
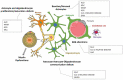
Astrocytes are very versatile cells, endowed with multitasking capacities to ensure brain homeostasis maintenance from brain development to adult life. It has become increasingly evident that astrocytes play a central role in many central nervous system pathologies, not only as regulators of defensive responses against brain insults but also as primary culprits of the disease onset and progression. This is particularly evident in some rare leukodystrophies (LDs) where white matter/myelin deterioration is due to primary astrocyte dysfunctions. Understanding the molecular defects causing these LDs may help clarify astrocyte contribution to myelin formation/maintenance and favor the identification of possible therapeutic targets for LDs and other CNS demyelinating diseases. To date, the pathogenic mechanisms of these LDs are poorly known due to the rarity of the pathological tissue and the failure of the animal models to fully recapitulate the human diseases. Thus, the development of human induced pluripotent stem cells (hiPSC) from patient fibroblasts and their differentiation into astrocytes is a promising approach to overcome these issues. In this review, we discuss the primary role of astrocytes in LD pathogenesis, the experimental models currently available and the advantages, future evolutions, perspectives, and limitations of hiPSC to study pathologies implying astrocyte dysfunctions.
Keywords: 3D models; Aicardi–Goutières; AxD; MLC; VWM; glial cells; human astrocytes; myelin; stem cells; white matter.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




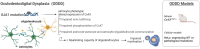



References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

