CRISPR/Cas9 Genome Editing vs. Over-Expression for Fluorescent Extracellular Vesicle-Labeling: A Quantitative Analysis
- PMID: 35008709
- PMCID: PMC8745383
- DOI: 10.3390/ijms23010282
CRISPR/Cas9 Genome Editing vs. Over-Expression for Fluorescent Extracellular Vesicle-Labeling: A Quantitative Analysis
Abstract
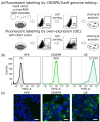
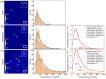
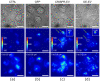
Over-expression of fluorescently-labeled markers for extracellular vesicles is frequently used to visualize vesicle up-take and transport. EVs that are labeled by over-expression show considerable heterogeneity regarding the number of fluorophores on single particles, which could potentially bias tracking and up-take studies in favor of more strongly-labeled particles. To avoid the potential artefacts that are caused by over-expression, we developed a genome editing approach for the fluorescent labeling of the extracellular vesicle marker CD63 with green fluorescent protein using the CRISPR/Cas9 technology. Using single-molecule sensitive fluorescence microscopy, we quantitatively compared the degree of labeling of secreted small extracellular vesicles from conventional over-expression and the CRISPR/Cas9 approach with true single-particle measurements. With our analysis, we can demonstrate a larger fraction of single-GFP-labeled EVs in the EVs that were isolated from CRISPR/Cas9-modified cells (83%) compared to EVs that were isolated from GFP-CD63 over-expressing cells (36%). Despite only single-GFP-labeling, CRISPR-EVs can be detected and discriminated from auto-fluorescence after their up-take into cells. To demonstrate the flexibility of the CRISPR/Cas9 genome editing method, we fluorescently labeled EVs using the HaloTag® with lipid membrane permeable dye, JaneliaFluor® 646, which allowed us to perform 3D-localization microscopy of single EVs taken up by the cultured cells.
Keywords: CD63; CRISPR/Cas9; atomic force microscopy; extracellular vesicles; genome editing; single-molecule fluorescence microscopy; single-molecule labeling stoichiometry.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures


References
-
- Yáñez-Mó M., Siljander P.R.-M., Andreu Z., Bedina Zavec A., Borràs F.E., Buzas E.I., Buzas K., Casal E., Cappello F., Carvalho J., et al. Biological properties of extracellular vesicles and their physiological functions. J. Extracell. Vesicles. 2015;4:27066. doi: 10.3402/jev.v4.27066. - DOI - PMC - PubMed
-
- Valcz G., Buzás E.I., Sebestyén A., Krenács T., Szállási Z., Igaz P., Molnár B. Extracellular Vesicle-Based Communication May Contribute to the Co-Evolution of Cancer Stem Cells and Cancer-Associated Fibroblasts in Anti-Cancer Therapy. Cancers. 2020;12:2324. doi: 10.3390/cancers12082324. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

