A Novel Role of BIRC3 in Stemness Reprogramming of Glioblastoma
- PMID: 35008722
- PMCID: PMC8745052
- DOI: 10.3390/ijms23010297
A Novel Role of BIRC3 in Stemness Reprogramming of Glioblastoma
Abstract
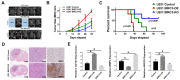
Stemness reprogramming remains a largely unaddressed principal cause of lethality in glioblastoma (GBM). It is therefore of utmost importance to identify and target mechanisms that are essential for GBM stemness and self-renewal. Previously, we implicated BIRC3 as an essential mediator of therapeutic resistance and survival adaptation in GBM. In this study, we present novel evidence that BIRC3 has an essential noncanonical role in GBM self-renewal and stemness reprogramming. We demonstrate that BIRC3 drives stemness reprogramming of human GBM cell lines, mouse GBM cell lines and patient-derived GBM stem cells (GSCs) through regulation of BMP4 signaling axis. Specifically, BIRC3 induces stemness reprogramming in GBM through downstream inactivation of BMP4 signaling. RNA-Seq interrogation of the stemness reprogramming hypoxic (pseudopalisading necrosis and perinecrosis) niche in GBM patient tissues further validated the high BIRC3/low BMP4 expression correlation. BIRC3 knockout upregulated BMP4 expression and prevented stemness reprogramming of GBM models. Furthermore, siRNA silencing of BMP4 restored stemness reprogramming of BIRC3 knockout in GBM models. In vivo silencing of BIRC3 suppressed tumor initiation and progression in GBM orthotopic intracranial xenografts. The stemness reprograming of both GSCs and non-GSCs populations highlights the impact of BIRC3 on intra-tumoral cellular heterogeneity GBM. Our study has identified a novel function of BIRC3 that can be targeted to reverse stemness programming of GBM.
Keywords: BIRC3; BMP4; GBM; brain tumor; cancer stem cell; stemness.
Conflict of interest statement
The authors declare that they have no competing interests, or any other interests that might be perceived to influence the results and/or discussion reported in this paper.
Figures

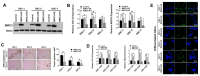


Similar articles
-
BIRC3 is a biomarker of mesenchymal habitat of glioblastoma, and a mediator of survival adaptation in hypoxia-driven glioblastoma habitats.Sci Rep. 2017 Aug 24;7(1):9350. doi: 10.1038/s41598-017-09503-8. Sci Rep. 2017. PMID: 28839258 Free PMC article.
-
Engagement of cellular prion protein with the co-chaperone Hsp70/90 organizing protein regulates the proliferation of glioblastoma stem-like cells.Stem Cell Res Ther. 2017 Apr 17;8(1):76. doi: 10.1186/s13287-017-0518-1. Stem Cell Res Ther. 2017. PMID: 28412969 Free PMC article.
-
Long non-coding RNA lung cancer-associated transcript-1 promotes glioblastoma progression by enhancing Hypoxia-inducible factor 1 alpha activity.Neuro Oncol. 2024 Aug 5;26(8):1388-1401. doi: 10.1093/neuonc/noae036. Neuro Oncol. 2024. PMID: 38456228 Free PMC article.
-
The Role of Mesenchymal Reprogramming in Malignant Clonal Evolution and Intra-Tumoral Heterogeneity in Glioblastoma.Cells. 2024 May 30;13(11):942. doi: 10.3390/cells13110942. Cells. 2024. PMID: 38891074 Free PMC article. Review.
-
Integrative analysis of cell adhesion molecules in glioblastoma identified prostaglandin F2 receptor inhibitor (PTGFRN) as an essential gene.BMC Cancer. 2022 Jun 11;22(1):642. doi: 10.1186/s12885-022-09682-2. BMC Cancer. 2022. PMID: 35690717 Free PMC article. Review.
Cited by
-
Astrocytes and the tumor microenvironment inflammatory state dictate the killing of glioblastoma cells by Smac mimetic compounds.Cell Death Dis. 2024 Aug 15;15(8):592. doi: 10.1038/s41419-024-06971-5. Cell Death Dis. 2024. PMID: 39147758 Free PMC article.
-
Epigenetic Activation of TUSC3 Sensitizes Glioblastoma to Temozolomide Independent of MGMT Promoter Methylation Status.Int J Mol Sci. 2023 Oct 14;24(20):15179. doi: 10.3390/ijms242015179. Int J Mol Sci. 2023. PMID: 37894860 Free PMC article.
-
Mechanism exploration and model construction for small cell transformation in EGFR-mutant lung adenocarcinomas.Signal Transduct Target Ther. 2024 Oct 2;9(1):261. doi: 10.1038/s41392-024-01981-3. Signal Transduct Target Ther. 2024. PMID: 39353908 Free PMC article.
-
SNHG1, a KLF4-upregulated gene, promotes glioma cell survival and tumorigenesis under endoplasmic reticulum stress by upregulating BIRC3 expression.J Cell Mol Med. 2023 Jul;27(13):1806-1819. doi: 10.1111/jcmm.17779. Epub 2023 May 26. J Cell Mol Med. 2023. PMID: 37243389 Free PMC article.
-
Delineating the glioblastoma stemness by genes involved in cytoskeletal rearrangements and metabolic alterations.World J Stem Cells. 2023 May 26;15(5):302-322. doi: 10.4252/wjsc.v15.i5.302. World J Stem Cells. 2023. PMID: 37342224 Free PMC article. Review.
References
-
- Alves A.L.V., Gomes I.N.F., Carloni A.C., Rosa M.N., da Silva L.S., Evangelista A.F., Reis R.M., Silva V.A.O. Role of glioblastoma stem cells in cancer therapeutic resistance: A perspective on antineoplastic agents from natural sources and chemical derivatives. Stem Cell Res. Ther. 2021;12:206. doi: 10.1186/s13287-021-02231-x. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

