Biochemical and Cellular Characterization of New Radio-Resistant Cell Lines Reveals a Role of Natural Flavonoids to Bypass Senescence
- PMID: 35008725
- PMCID: PMC8745286
- DOI: 10.3390/ijms23010301
Biochemical and Cellular Characterization of New Radio-Resistant Cell Lines Reveals a Role of Natural Flavonoids to Bypass Senescence
Abstract
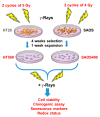
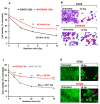
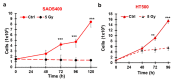
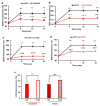
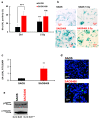
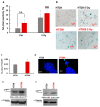
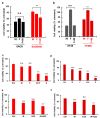
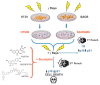
Cancer is one of the main causes of death worldwide, and, among the most frequent cancer types, osteosarcoma accounts for 56% of bone neoplasms observed in children and colorectal cancer for 10.2% of tumors diagnosed in the adult population. A common and frequent hurdle in cancer treatment is the emergence of resistance to chemo- and radiotherapy whose biological causes are largely unknown. In the present work, human osteosarcoma (SAOS) and colorectal adenocarcinoma (HT29) cell lines were γ-irradiated at doses mimicking the sub-lethal irradiation in clinical settings to obtain two radio-resistant cellular sub-populations named SAOS400 and HT500, respectively. Since "therapy-induced senescence" (TIS) is often associated with tumor response to radiotherapy in cancer cells, we measured specific cellular and biochemical markers of senescence in SAOS400 and HT500 cells. In detail, both cell lines were characterized by a higher level of expression of cyclin-dependent kinase inhibitors p16INK4 and p21CIP1 and increased positivity to SAβ-gal (senescence-associated β-galactosidase) with respect to parental cells. Moreover, the intracellular levels of reactive oxygen species in the resistant cells were significantly lower compared to the parental counterparts. Subsequently, we demonstrated that senolytic agents were able to sensitize SAOS400 and HT500 to cell death induced by γ-irradiation. Employing two natural flavonoids, fisetin and quercetin, and a BH3-mimetic, ABT-263/navitoclax, we observed that their association with γ-irradiation significantly reduced the expression of p16INK4, p21CIP1 and synergistically (combination index < 1) increased cell death compared to radiation mono-alone treatments. The present results reinforce the potential role of senolytics as adjuvant agents in cancer therapy.
Keywords: BH3 mimetics; cancer therapy; flavonoids; senolytics; therapy-induced senescence; γ radiation resistance.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Carotenoid-Enriched Nanoemulsions and γ-Rays Synergistically Induce Cell Death in a Novel Radioresistant Osteosarcoma Cell Line.Int J Mol Sci. 2022 Dec 15;23(24):15959. doi: 10.3390/ijms232415959. Int J Mol Sci. 2022. PMID: 36555605 Free PMC article.
-
Specific Irreversible Cell-Cycle Arrest and Depletion of Cancer Cells Obtained by Combining Curcumin and the Flavonoids Quercetin and Fisetin.Genes (Basel). 2022 Jun 23;13(7):1125. doi: 10.3390/genes13071125. Genes (Basel). 2022. PMID: 35885908 Free PMC article.
-
Senolytic Flavonoids Enhance Type-I and Type-II Cell Death in Human Radioresistant Colon Cancer Cells through AMPK/MAPK Pathway.Cancers (Basel). 2023 May 8;15(9):2660. doi: 10.3390/cancers15092660. Cancers (Basel). 2023. PMID: 37174126 Free PMC article.
-
Nanocarriers for natural polyphenol senotherapeutics.Aging Cell. 2024 May;23(5):e14178. doi: 10.1111/acel.14178. Epub 2024 Apr 29. Aging Cell. 2024. PMID: 38685568 Free PMC article. Review.
-
Potential Role of Polyphenolic Flavonoids as Senotherapeutic Agents in Degenerative Diseases and Geroprotection.Pharmaceut Med. 2022 Dec;36(6):331-352. doi: 10.1007/s40290-022-00444-w. Epub 2022 Sep 13. Pharmaceut Med. 2022. PMID: 36100824 Free PMC article. Review.
Cited by
-
An organic extract from ascidian Ciona robusta induces cytotoxic autophagy in human malignant cell lines.Front Chem. 2024 Feb 8;12:1322558. doi: 10.3389/fchem.2024.1322558. eCollection 2024. Front Chem. 2024. PMID: 38389727 Free PMC article.
-
Melatonin: a potential target for regulating ovarian function.Arch Gynecol Obstet. 2025 Sep;312(3):721-731. doi: 10.1007/s00404-025-08079-3. Epub 2025 Jun 8. Arch Gynecol Obstet. 2025. PMID: 40483629 Free PMC article. Review.
-
The development of radioresistant oral squamous carcinoma cell lines and identification of radiotherapy-related biomarkers.Clin Transl Oncol. 2023 Oct;25(10):3006-3020. doi: 10.1007/s12094-023-03169-7. Epub 2023 Apr 7. Clin Transl Oncol. 2023. PMID: 37029240
-
The Role of Radiosensitizing Drugs in Osteosarcoma Treatment: Mechanisms and Clinical Perspectives.Drug Des Devel Ther. 2025 Mar 14;19:1927-1942. doi: 10.2147/DDDT.S512479. eCollection 2025. Drug Des Devel Ther. 2025. PMID: 40110500 Free PMC article. Review.
-
Carotenoid-Enriched Nanoemulsions and γ-Rays Synergistically Induce Cell Death in a Novel Radioresistant Osteosarcoma Cell Line.Int J Mol Sci. 2022 Dec 15;23(24):15959. doi: 10.3390/ijms232415959. Int J Mol Sci. 2022. PMID: 36555605 Free PMC article.
References
-
- Perego M., Tyurin V.A., Tyurina Y.Y., Yellets J., Nacarelli T., Lin C., Nefedova Y., Kossenkov A., Liu Q., Sreedhar S., et al. Reactivation of dormant tumor cells by modified lipids derived from stress-activated neutrophils. Sci. Transl. Med. 2020;12 doi: 10.1126/scitranslmed.abb5817. - DOI - PMC - PubMed
-
- Kim E.H., Kim M.S., Lee K.H., Sai S., Jeong Y.K., Koh J.S., Kong C.B. Effect of low- and high-linear energy transfer radiation on in vitro and orthotopic in vivo models of osteosarcoma by activation of caspase-3 and -9. Int. J. Oncol. 2017;51:1124–1134. doi: 10.3892/ijo.2017.4102. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

