Availability of Arg, but Not tRNA, Is a Rate-Limiting Factor for Intracellular Arginylation
- PMID: 35008737
- PMCID: PMC8745564
- DOI: 10.3390/ijms23010314
Availability of Arg, but Not tRNA, Is a Rate-Limiting Factor for Intracellular Arginylation
Abstract
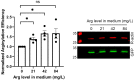
Protein arginylation, mediated by arginyltransferase ATE1, is a posttranslational modification of emerging biological importance that consists of transfer of the amino acid Arg from tRNA to protein and peptide targets. ATE1 can bind tRNA and exhibits specificity toward particular tRNA types, but its dependence on the availability of the major components of the arginylation reaction has never been explored. Here we investigated key intracellular factors that can potentially regulate arginylation in vivo, including several tRNA types that show strong binding to ATE1, as well as availability of free Arg, in an attempt to identify intracellular rate limiting steps for this enzyme. Our results demonstrate that, while modulation of tRNA levels in cells does not lead to any changes in intracellular arginylation efficiency, availability of free Arg is a potentially rate-limiting factor that facilitates arginylation if added to the cultured cells. Our results broadly outline global pathways that may be involved in the regulation of arginylation in vivo.
Keywords: arginine; arginine metabolism; arginylation; arginyltrasferase; tRNA.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Wang J., Han X., Wong C.C., Cheng H., Aslanian A., Xu T., Leavis P., Roder H., Hedstrom L., Yates J.R., et al. Arginyltransferase ATE1 Catalyzes Midchain Arginylation of Proteins at Side Chain Carboxylates In Vivo. Chem. Biol. 2014;21:331–337. doi: 10.1016/j.chembiol.2013.12.017. - DOI - PMC - PubMed
-
- Rai R., Wong C.C.L., Xu T., Leu N.A., Dong D.W., Guo C., McLaughlin K.J., Yates J.R., III, Kashina A. Arginyltransferase regulates alpha cardiac actin function, myofibril formation and contractility during heart development. Development. 2008;135:3881–3889. doi: 10.1242/dev.022723. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

