Molecular Structure of Nickel Octamethylporphyrin-Rare Experimental Evidence of a Ruffling Effect in Gas Phase
- PMID: 35008747
- PMCID: PMC8745403
- DOI: 10.3390/ijms23010320
Molecular Structure of Nickel Octamethylporphyrin-Rare Experimental Evidence of a Ruffling Effect in Gas Phase
Abstract
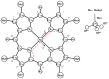
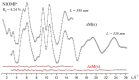
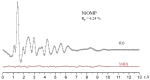
The structure of a free nickel (II) octamethylporphyrin (NiOMP) molecule was determined for the first time through a combined gas-phase electron diffraction (GED) and mass spectrometry (MS) experiment, as well as through quantum chemical (QC) calculations. Density functional theory (DFT) calculations do not provide an unambiguous answer about the planarity or non-planar distortion of the NiOMP skeleton. The GED refinement in such cases is non-trivial. Several approaches to the inverse problem solution were used. The obtained results allow us to argue that the ruffling effect is manifested in the NiOMP molecule. The minimal critical distance between the central atom of the metal and nitrogen atoms of the coordination cavity that provokes ruffling distortion in metal porphyrins is about 1.96 Å.
Keywords: electron diffraction; molecular structure; nickel; porphyrin; quantum chemistry; ruffling distortion.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
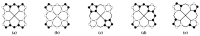



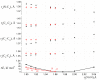

References
-
- Kalyanasundaram K. Photochemistry of Polypyridine and Porphyrin Complexes. Academic Press; London, UK: 1992.
-
- Berezin B.D., Enikolopyan N.S. Metalloporphyrins. Nauka; Moscow, Russia: 1988. (In Russian)
-
- Askarov K.A., Berezin B.D., Bystritskaya E.V., Golubchikov O.A., Koifman O.I., Kuzmitsky V.A., Mairanovsky V.G., Ponomarev N.V., Rish M.A., Smirnov B.R., et al. In: Porphyrins: Spectroscopy, Electrochemistry, Application. Enikolopyan N.S., editor. Nauka; Moscow, Russia: 1987. in Russian.
-
- Mao H., Deng H., Li H., Shen Y., Lu Z., Xu H. Photosensitization of TiO2 semiconductor with porphyrin. J. Photochem. Photobiol. A Chem. 1998;114:209–212. doi: 10.1016/S1010-6030(97)00260-8. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

