Extracellular Vesicles Mediate Communication between Endothelial and Vascular Smooth Muscle Cells
- PMID: 35008757
- PMCID: PMC8745747
- DOI: 10.3390/ijms23010331
Extracellular Vesicles Mediate Communication between Endothelial and Vascular Smooth Muscle Cells
Abstract
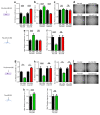
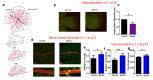
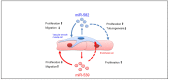
The recruitment of pericytes and vascular smooth muscle cells (SMCs) that enwrap endothelial cells (ECs) is a crucial process for vascular maturation and stabilization. Communication between these two cell types is crucial during vascular development and in maintaining vessel homeostasis. Extracellular vesicles (EVs) have emerged as a new communication tool involving the exchange of microRNAs between cells. In the present study, we searched for microRNAs that could be transferred via EVs from ECs to SMCs and vice versa. Thanks to a microRNA profiling experiment, we found that two microRNAs are more exported in each cell type in coculture experiments: while miR-539 is more secreted by ECs, miR-582 is more present in EVs from SMCs. Functional assays revealed that both microRNAs can modulate both cell-type phenotypes. We further identified miR-539 and miR-582 targets, in agreement with their respective cell functions. The results obtained in vivo in the neovascularization model suggest that miR-539 and miR-582 might cooperate to trigger the process of blood vessel coverage by smooth muscle cells in a mature plexus. Taken together, these results are the first to highlight the role of miR-539 and miR-582 in angiogenesis and communication between ECs and SMCs.
Keywords: angiogenesis; exosome; extracellular vesicle; miR-539; miR-582; microRNA.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

