Immunopathological Analysis of a Mouse Model of Arthritis-Associated Scleritis and Implications for Molecular Targeted Therapy for Severe Scleritis
- PMID: 35008766
- PMCID: PMC8745222
- DOI: 10.3390/ijms23010341
Immunopathological Analysis of a Mouse Model of Arthritis-Associated Scleritis and Implications for Molecular Targeted Therapy for Severe Scleritis
Abstract
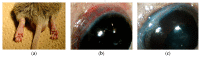
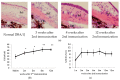
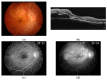
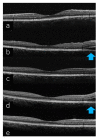
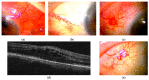
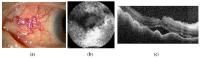
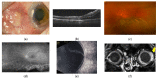
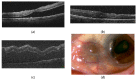
Scleritis involves inflammation of the sclera, which constitutes 75% of the wall of the eye. This pathology is often seen as an ocular lesion associated with systemic inflammatory diseases. Severe types of scleritis such as posterior scleritis require urgent immunosuppressive treatments, including molecularly targeted therapies to avoid permanent visual impairment. Which molecules should be selected as targets has remained unclear. To clarify the pathogenesis of scleritis and propose appropriate target molecules for therapy, we have established novel animal model of scleritis by modifying the Collagen-II Induced Arthritis (CIA) model. Immunization twice with collagen II emulsified with complete Freund's adjuvant (CFA) caused arthritis and scleritis. The clinical appearance resembled human diffuse scleritis. Histopathological analysis suggested that macrophages, plasma cells, deposition of immune complexes, and growth of blood and lymphatic vessels are involved in the pathogenesis of CIA-associated scleritis. In addition, we analysed the background diseases of posterior scleritis and responses to molecularly targeted therapies as a case series study. We inferred from both the animal model and case series study that targets should not be T cells, but factors inhibiting macrophage activity such as tumor necrosis factor (TNF) and interleukin (IL)-6, and molecules suppressing antibody-producing cells such as CD20 on B cells should be targeted by molecularly targeted therapies.
Keywords: CTLA4Ig; IL-6 inhibitors; TNF inhibitors; animal model; anti-CD20; biologic agent; collagen-II induced arthritis; molecular targeted therapy; posterior scleritis; scleritis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Protection against cartilage and bone destruction by systemic interleukin-4 treatment in established murine type II collagen-induced arthritis.Arthritis Res. 1999;1(1):81-91. doi: 10.1186/ar14. Epub 1999 Oct 26. Arthritis Res. 1999. PMID: 11056663 Free PMC article.
-
Anti-arthritic and anti-inflammatory effects of (-)-Epicatechin-3-O-β-d-allopyranoside, a constituent of Davallia formosana.Phytomedicine. 2019 Jan;52:12-22. doi: 10.1016/j.phymed.2018.09.192. Epub 2018 Sep 18. Phytomedicine. 2019. PMID: 30599891
-
The prothrombinase activity of FGL2 contributes to the pathogenesis of experimental arthritis.Scand J Rheumatol. 2011;40(4):269-78. doi: 10.3109/03009742.2010.536163. Epub 2011 Apr 6. Scand J Rheumatol. 2011. PMID: 21469939
-
Biologic Therapies and Small Molecules for the Management of Non-Infectious Scleritis: A Narrative Review.Ophthalmol Ther. 2021 Dec;10(4):777-813. doi: 10.1007/s40123-021-00393-8. Epub 2021 Sep 2. Ophthalmol Ther. 2021. PMID: 34476773 Free PMC article. Review.
-
Scleritis: Immunopathogenesis and molecular basis for therapy.Prog Retin Eye Res. 2013 Jul;35:44-62. doi: 10.1016/j.preteyeres.2013.02.004. Epub 2013 Feb 26. Prog Retin Eye Res. 2013. PMID: 23454614 Review.
Cited by
-
Adalimumab plus Conventional Therapy versus Conventional Therapy in Refractory Non-Infectious Scleritis.J Clin Med. 2022 Nov 11;11(22):6686. doi: 10.3390/jcm11226686. J Clin Med. 2022. PMID: 36431163 Free PMC article.
-
Comparison of Intestinal Microbes in Noninfectious Anterior Scleritis Patients With and Without Rheumatoid Arthritis.Front Microbiol. 2022 Jun 9;13:925929. doi: 10.3389/fmicb.2022.925929. eCollection 2022. Front Microbiol. 2022. PMID: 35756002 Free PMC article.
-
Scleral Proteome in Noninfectious Scleritis Unravels Upregulation of Filaggrin-2 and Signs of Neovascularization.Invest Ophthalmol Vis Sci. 2023 Mar 1;64(3):27. doi: 10.1167/iovs.64.3.27. Invest Ophthalmol Vis Sci. 2023. PMID: 36930145 Free PMC article.
-
Potential Biomarkers for Noninfectious Scleritis Identified by Serum and Tear Fluid Proteomics.Ophthalmol Sci. 2023 Oct 5;4(1):100407. doi: 10.1016/j.xops.2023.100407. eCollection 2024 Jan-Feb. Ophthalmol Sci. 2023. PMID: 38054106 Free PMC article.
-
Pathogenesis of Extraarticular Manifestations in Rheumatoid Arthritis-A Comprehensive Review.Biomedicines. 2023 Apr 24;11(5):1262. doi: 10.3390/biomedicines11051262. Biomedicines. 2023. PMID: 37238933 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

