Microporous Carbon and Carbon/Metal Composite Materials Derived from Bio-Benzoxazine-Linked Precursor for CO2 Capture and Energy Storage Applications
- PMID: 35008773
- PMCID: PMC8745757
- DOI: 10.3390/ijms23010347
Microporous Carbon and Carbon/Metal Composite Materials Derived from Bio-Benzoxazine-Linked Precursor for CO2 Capture and Energy Storage Applications
Abstract
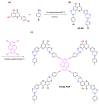
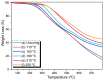
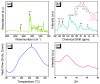
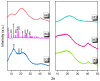
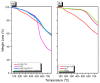
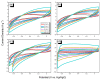
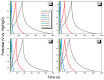
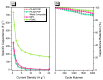
There is currently a pursuit of synthetic approaches for designing porous carbon materials with selective CO2 capture and/or excellent energy storage performance that significantly impacts the environment and the sustainable development of circular economy. In this study we prepared a new bio-based benzoxazine (AP-BZ) in high yield through Mannich condensation of apigenin, a naturally occurring phenol, with 4-bromoaniline and paraformaldehyde. We then prepared a PA-BZ porous organic polymer (POP) through Sonogashira coupling of AP-BZ with 1,3,6,8-tetraethynylpyrene (P-T) in the presence of Pd(PPh3)4. In situ Fourier transform infrared spectroscopy and differential scanning calorimetry revealed details of the thermal polymerization of the oxazine rings in the AP-BZ monomer and in the PA-BZ POP. Next, we prepared a microporous carbon/metal composite (PCMC) in three steps: Sonogashira coupling of AP-BZ with P-T in the presence of a zeolitic imidazolate framework (ZIF-67) as a directing hard template, affording a PA-BZ POP/ZIF-67 composite; etching in acetic acid; and pyrolysis of the resulting PA-BZ POP/metal composite at 500 °C. Powder X-ray diffraction, thermogravimetric analysis, scanning electron microscopy, transmission electron microscopy, and Brunauer-Emmett-Teller (BET) measurements revealed the properties of the as-prepared PCMC. The PCMC material exhibited outstanding thermal stability (Td10 = 660 °C and char yield = 75 wt%), a high BET surface area (1110 m2 g-1), high CO2 adsorption (5.40 mmol g-1 at 273 K), excellent capacitance (735 F g-1), and a capacitance retention of up to 95% after 2000 galvanostatic charge-discharge (GCD) cycles; these characteristics were excellent when compared with those of the corresponding microporous carbon (MPC) prepared through pyrolysis of the PA-BZ POP precursors with a ZIF-67 template at 500 °C.
Keywords: energy storage; polybenzoxazine; porous organic polymers; ring-opening polymerization; zeolitic imidazolate frameworks.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Deliballi Z., Kiskan B., Yagci Y. Light induced crosslinking of main chain polybenzoxazines. Polym. Chem. 2021;12:5781–5786. doi: 10.1039/D1PY01080H. - DOI
-
- Lu Y., Yu X., Evans C.J., Yang S., Zhang K. Elucidating the role of acetylene in ortho-phthalimide functional benzoxazines: Design, synthesis, and structure–property investigations. Polym. Chem. 2021;12:5059–5068. doi: 10.1039/D1PY00850A. - DOI
-
- Aly K.I., Mohamed M.G., Younis O., Mahross M.H., Hakim M.A., Sayed M.M. Salicylaldehyde azine-functionalized polybenzoxazine: Synthesis, characterization, and its nanocomposites as coatings for inhibiting the mild steel corrosion. Prog. Org. Coat. 2020;138:105385. doi: 10.1016/j.porgcoat.2019.105385. - DOI
-
- Zhang X., Mohamed M.G., Xin Z., Kuo S.W. A tetraphenylethylene-functionalized benzoxazine and copper(II) acetylacetonate form a high-performance polybenzoxazine. Polymer. 2020;201:122552. doi: 10.1016/j.polymer.2020.122552. - DOI
-
- Mohamed M.G., Kuo S.W., Mahdy A., Ghayd I.M., Aly K.I. Bisbenzylidene cyclopentanone and cyclohexanone-functionalized polybenzoxazine nanocomposites: Synthesis, characterization, and use for corrosion protection on mild steel. Mater. Today Commun. 2020;25:101418. doi: 10.1016/j.mtcomm.2020.101418. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

