Synthesis of 3-Amino-4-substituted Monocyclic ß-Lactams-Important Structural Motifs in Medicinal Chemistry
- PMID: 35008788
- PMCID: PMC8745335
- DOI: 10.3390/ijms23010360
Synthesis of 3-Amino-4-substituted Monocyclic ß-Lactams-Important Structural Motifs in Medicinal Chemistry
Abstract
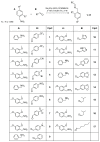
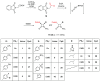
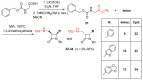
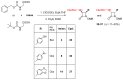
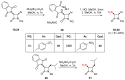
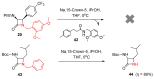
Monocyclic ß-lactams (azetidin-2-ones) exhibit a wide range of biological activities, the most important of which are antibacterial, anticancer, and cholesterol absorption inhibitory activities. The synthesis of decorated monocyclic ß-lactams is challenging because their ring is highly constrained and consequently reactive, which is also an important determinant of their biological activity. We present the optimized synthesis of orthogonally protected 3-amino-4-substituted monocyclic ß-lactams. Among several possible synthetic approaches, Staudinger cycloaddition proved to be the most promising method for initial ring formation, yielding monocyclic ß-lactams with different substituents at the C-4 position, a phthalimido-protected 3-amino group, and a (dimethoxy)benzyl protected ring nitrogen. Challenging deprotection methods were then investigated. Oxidative cleavage with cerium ammonium nitrate and ammonia-free Birch reduction was found to be most effective for selective removal of ring nitrogen protection. Hydrazine hydrate was used for deprotection of the phthalimido group, and the procedure had to be modified by the addition of HCl in the case of aromatic substituents at the C-4 position. The presented methods and the synthesized 3-amino-4-substituted monocyclic ß-lactam derivatives are an important step toward new ß-lactams with potential pharmacological activities.
Keywords: 3-amino-4-substituted azetidin-2-one; Staudinger [2+2] cyclocondensation; cerium ammonium nitrate; hydrazine hydrate; monocyclic ß-lactam.
Conflict of interest statement
Authors declare no conflict of interest.
Figures



Similar articles
-
Synthesis and biochemical evaluation of new 3-amido-4-substituted monocyclic ß-lactams as inhibitors of penicillin-binding protein(s).Acta Pharm. 2024 Sep 14;74(3):423-440. doi: 10.2478/acph-2024-0024. Print 2024 Sep 1. Acta Pharm. 2024. PMID: 39279527
-
Studies on monocyclic beta-lactam antibiotics. III. Synthesis and antibacterial activity of N-(aromatic heterocyclic substituted)azetidin-2-ones.J Antibiot (Tokyo). 1986 Jan;39(1):76-89. doi: 10.7164/antibiotics.39.76. J Antibiot (Tokyo). 1986. PMID: 3949632
-
Synthesis and Antibacterial Properties of 3-Amino-β-Lactams Bearing a Heteroatom-Containing C4 Substituent.ChemMedChem. 2025 May 5;20(9):e202400994. doi: 10.1002/cmdc.202400994. Epub 2025 Mar 16. ChemMedChem. 2025. PMID: 39906996 Review.
-
Repurposing of monocyclic β-lactams as anti-inflammatory agents - The case of new ferrocene-azetidin-2-one hybrids.Eur J Med Chem. 2024 Dec 15;280:116910. doi: 10.1016/j.ejmech.2024.116910. Epub 2024 Oct 1. Eur J Med Chem. 2024. PMID: 39406117
-
Azetidin-2-ones, synthon for biologically important compounds.Curr Med Chem. 2004 Jul;11(14):1889-920. doi: 10.2174/0929867043364874. Curr Med Chem. 2004. PMID: 15279573 Review.
Cited by
-
Copper-Catalyzed Enantioconvergent Radical C(sp3)-N Cross-Coupling to Access Chiral α-Amino-β-lactams.Precis Chem. 2023 Oct 10;1(10):576-582. doi: 10.1021/prechem.3c00084. eCollection 2023 Dec 25. Precis Chem. 2023. PMID: 39473577 Free PMC article.
References
-
- World Health Organization . WHO Report on Surveillance of Antibiotic Consumption: 2016–2018 Early Implementation. World Health Organization; Geneva, Switzerland: 2018.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

