Iron-Chelation Treatment by Novel Thiosemicarbazone Targets Major Signaling Pathways in Neuroblastoma
- PMID: 35008802
- PMCID: PMC8745636
- DOI: 10.3390/ijms23010376
Iron-Chelation Treatment by Novel Thiosemicarbazone Targets Major Signaling Pathways in Neuroblastoma
Abstract
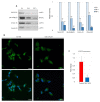
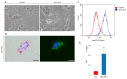
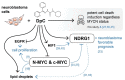
Despite constant advances in the field of pediatric oncology, the survival rate of high-risk neuroblastoma patients remains poor. The molecular and genetic features of neuroblastoma, such as MYCN amplification and stemness status, have established themselves not only as potent prognostic and predictive factors but also as intriguing targets for personalized therapy. Novel thiosemicarbazones target both total level and activity of a number of proteins involved in some of the most important signaling pathways in neuroblastoma. In this study, we found that di-2-pyridylketone 4-cyclohexyl-4-methyl-3-thiosemicarbazone (DpC) potently decreases N-MYC in MYCN-amplified and c-MYC in MYCN-nonamplified neuroblastoma cell lines. Furthermore, DpC succeeded in downregulating total EGFR and phosphorylation of its most prominent tyrosine residues through the involvement of NDRG1, a positive prognostic marker in neuroblastoma, which was markedly upregulated after thiosemicarbazone treatment. These findings could provide useful knowledge for the treatment of MYC-driven neuroblastomas that are unresponsive to conventional therapies.
Keywords: DpC; EGFR; MYC; NDRG1; lipid droplet; neuroblastoma; thiosemicarbazone.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- Nuchtern J.G., London W.B., Barnewolt C.E., Naranjo A., McGrady P.W., Geiger J.D., Diller L., Schmidt M., Lou Maris J.M., Cohn S.L. et al. A prospective study of expectant observation as primary therapy for neuroblastoma in young infants: A children’s oncology group study. Ann. Surg. 2012;256:573. doi: 10.1097/SLA.0b013e31826cbbbd. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

