Citrus Naringenin Increases Neuron Survival in Optic Nerve Crush Injury Model by Inhibiting JNK-JUN Pathway
- PMID: 35008811
- PMCID: PMC8745540
- DOI: 10.3390/ijms23010385
Citrus Naringenin Increases Neuron Survival in Optic Nerve Crush Injury Model by Inhibiting JNK-JUN Pathway
Abstract
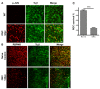
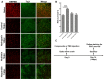
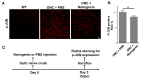
Traumatic nerve injury activates cell stress pathways, resulting in neuronal death and loss of vital neural functions. To date, there are no available neuroprotectants for the treatment of traumatic neural injuries. Here, we studied three important flavanones of citrus components, in vitro and in vivo, to reveal their roles in inhibiting the JNK (c-Jun N-terminal kinase)-JUN pathway and their neuroprotective effects in the optic nerve crush injury model, a kind of traumatic nerve injury in the central nervous system. Results showed that both neural injury in vivo and cell stress in vitro activated the JNK-JUN pathway and increased JUN phosphorylation. We also demonstrated that naringenin treatment completely inhibited stress-induced JUN phosphorylation in cultured cells, whereas nobiletin and hesperidin only partially inhibited JUN phosphorylation. Neuroprotection studies in optic nerve crush injury mouse models revealed that naringenin treatment increased the survival of retinal ganglion cells after traumatic optic nerve injury, while the other two components had no neuroprotective effect. The neuroprotection effect of naringenin was due to the inhibition of JUN phosphorylation in crush-injured retinal ganglion cells. Therefore, the citrus component naringenin provides neuroprotection through the inhibition of the JNK-JUN pathway by inhibiting JUN phosphorylation, indicating the potential application of citrus chemical components in the clinical therapy of traumatic optic nerve injuries.
Keywords: JNK-JUN pathway; JUN phosphorylation; naringenin; neuroprotection; optic nerve crush; retinal ganglion cells; traumatic nerve injury.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Mkk4 and Mkk7 are important for retinal development and axonal injury-induced retinal ganglion cell death.Cell Death Dis. 2018 Oct 26;9(11):1095. doi: 10.1038/s41419-018-1079-7. Cell Death Dis. 2018. PMID: 30367030 Free PMC article.
-
Role of tumor necrosis factor receptor-1 in the death of retinal ganglion cells following optic nerve crush injury in mice.Brain Res. 2004 Jan 23;996(2):202-12. doi: 10.1016/j.brainres.2003.10.029. Brain Res. 2004. PMID: 14697498
-
The transcription factor c-jun is activated in retinal ganglion cells in experimental rat glaucoma.Exp Eye Res. 2005 May;80(5):663-70. doi: 10.1016/j.exer.2004.11.016. Epub 2005 Jan 4. Exp Eye Res. 2005. PMID: 15862173
-
Naringenin: its chemistry and roles in neuroprotection.Nutr Neurosci. 2024 Jun;27(6):637-666. doi: 10.1080/1028415X.2023.2243089. Epub 2023 Aug 10. Nutr Neurosci. 2024. PMID: 37585716 Review.
-
A Scoping Review of the Skeletal Effects of Naringenin.Nutrients. 2022 Nov 16;14(22):4851. doi: 10.3390/nu14224851. Nutrients. 2022. PMID: 36432535 Free PMC article.
Cited by
-
Optic nerve injury models under varying forces.Int Ophthalmol. 2023 Mar;43(3):757-769. doi: 10.1007/s10792-022-02476-2. Epub 2022 Aug 29. Int Ophthalmol. 2023. PMID: 36038691 Free PMC article.
-
Citrus By-Products as a Valuable Source of Biologically Active Compounds with Promising Pharmaceutical, Biological and Biomedical Potential.Pharmaceuticals (Basel). 2023 Jul 29;16(8):1081. doi: 10.3390/ph16081081. Pharmaceuticals (Basel). 2023. PMID: 37630996 Free PMC article. Review.
-
Intravitreal injection of Huperzine A promotes retinal ganglion cells survival and axonal regeneration after optic nerve crush.Front Cell Neurosci. 2023 May 24;17:1145574. doi: 10.3389/fncel.2023.1145574. eCollection 2023. Front Cell Neurosci. 2023. PMID: 37293627 Free PMC article.
-
Exploring the mechanism of Si-Ni-San against depression by UPLC-Q-TOF-MS/MS integrated with network pharmacology: experimental research.Ann Med Surg (Lond). 2023 Nov 7;86(1):172-189. doi: 10.1097/MS9.0000000000001464. eCollection 2024 Jan. Ann Med Surg (Lond). 2023. PMID: 38222693 Free PMC article.
References
-
- Zhao S., Liu Z., Wang M., He D., Liu L., Shu Y., Song Z., Li H., Liu Y., Lu A. Anti-inflammatory effects of Zhishi and Zhiqiao revealed by network pharmacology integrated with molecular mechanism and metabolomics studies. Phytomedicine. 2018;50:61–72. doi: 10.1016/j.phymed.2018.09.184. - DOI - PubMed
-
- Tiza N.U., Thato M., Raymond D., Jeremy K., Burtram C.F. Additive antibacterial activity of naringenin and antibiotic combinations against multidrug resistant Staphylococcus aureus. Afr. J. Microbiol. Res. 2015;9:1513–1518. doi: 10.5897/AJMR2015.7514. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

