Platelet Mitochondrial Bioenergetics Reprogramming in Patients with Urothelial Carcinoma
- PMID: 35008814
- PMCID: PMC8745267
- DOI: 10.3390/ijms23010388
Platelet Mitochondrial Bioenergetics Reprogramming in Patients with Urothelial Carcinoma
Abstract
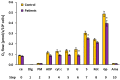
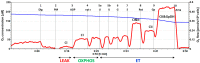
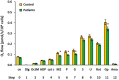
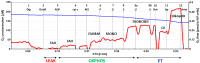
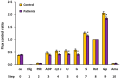
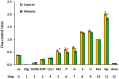
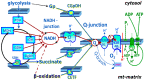
Mitochondrial bioenergetics reprogramming is an essential response of cells to stress. Platelets, an accessible source of mitochondria, have a crucial role in cancer development; however, the platelet mitochondrial function has not been studied in urothelial carcinoma (UC) patients. A total of 15 patients with UC and 15 healthy controls were included in the study. Parameters of platelet mitochondrial respiration were evaluated using the high-resolution respirometry method, and the selected antioxidant levels were determined by HPLC. In addition, oxidative stress was evaluated by the thiobarbituric acid reactive substances (TBARS) concentration in plasma. We demonstrated deficient platelet mitochondrial respiratory chain functions, oxidative phosphorylation (OXPHOS), and electron transfer (ET) capacity with complex I (CI)-linked substrates, and reduced the endogenous platelet coenzyme Q10 (CoQ10) concentration in UC patients. The activity of citrate synthase was decreased in UC patients vs. controls (p = 0.0191). γ-tocopherol, α-tocopherol in platelets, and β-carotene in plasma were significantly lower in UC patients (p = 0.0019; p = 0.02; p = 0.0387, respectively), whereas the plasma concentration of TBARS was increased (p = 0.0022) vs. controls. The changes in platelet mitochondrial bioenergetics are consistent with cell metabolism reprogramming in UC patients. We suppose that increased oxidative stress, decreased OXPHOS, and a reduced platelet endogenous CoQ10 level can contribute to the reprogramming of platelet mitochondrial OXPHOS toward the activation of glycolysis. The impaired mitochondrial function can contribute to increased oxidative stress by triggering the reverse electron transport from the CoQ10 cycle (Q-junction) to CI.
Keywords: mitochondrial bioenergetics; oxidative stress; platelets; reprogramming; urothelial carcinoma.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Platelet Mitochondrial Function and Endogenous Coenzyme Q10 Levels Could Be Used as Markers of Mitochondrial Health in Infertile Men: A Pilot Study.Int J Mol Sci. 2024 Dec 31;26(1):268. doi: 10.3390/ijms26010268. Int J Mol Sci. 2024. PMID: 39796125 Free PMC article.
-
The Effect of Adjuvant Therapy with Molecular Hydrogen on Endogenous Coenzyme Q10 Levels and Platelet Mitochondrial Bioenergetics in Patients with Non-Alcoholic Fatty Liver Disease.Int J Mol Sci. 2023 Aug 5;24(15):12477. doi: 10.3390/ijms241512477. Int J Mol Sci. 2023. PMID: 37569850 Free PMC article.
-
Platelet mitochondrial respiration and coenzyme Q10 could be used as new diagnostic strategy for mitochondrial dysfunction in rheumatoid diseases.PLoS One. 2021 Sep 28;16(9):e0256135. doi: 10.1371/journal.pone.0256135. eCollection 2021. PLoS One. 2021. PMID: 34582480 Free PMC article.
-
Perspective targeted diagnosis and therapy of mitochondrial bioenergetics across different diagnoses.Bratisl Lek Listy. 2024;125(11):693-700. doi: 10.4149/BLL_2024_105. Bratisl Lek Listy. 2024. PMID: 39487838 Review.
-
Mitochondrial bioenergetics in aging.Biochim Biophys Acta. 2000 Aug 15;1459(2-3):397-404. doi: 10.1016/s0005-2728(00)00177-8. Biochim Biophys Acta. 2000. PMID: 11004456 Review.
Cited by
-
Effect of Vaccination on Platelet Mitochondrial Bioenergy Function of Patients with Post-Acute COVID-19.Viruses. 2023 Apr 28;15(5):1085. doi: 10.3390/v15051085. Viruses. 2023. PMID: 37243171 Free PMC article.
-
Mass Spectrometry-Based Proteomics Analysis Unveils PTPRS Inhibits Proliferation and Inflammatory Response of Keratinocytes in Psoriasis.Inflammation. 2025 Feb;48(1):89-103. doi: 10.1007/s10753-024-02044-z. Epub 2024 May 13. Inflammation. 2025. PMID: 38739342
-
Elevated LDL-C, high blood pressure, and low peak O2 associate with platelet mitochondria function in children-The Arkansas Active Kids Study.Front Mol Biosci. 2023 Mar 22;10:1136975. doi: 10.3389/fmolb.2023.1136975. eCollection 2023. Front Mol Biosci. 2023. PMID: 37033448 Free PMC article.
-
WTAP mediated m6A-modified circ_0056856 contributes to the proliferation, migration, and invasion of IL-22-stimulated human keratinocyte by miR-197-3p/CDK1 axis.Arch Dermatol Res. 2024 May 24;316(6):208. doi: 10.1007/s00403-024-03097-8. Arch Dermatol Res. 2024. PMID: 38787443
-
Platelets in the tumor microenvironment and their biological effects on cancer hallmarks.Front Oncol. 2023 Mar 3;13:1121401. doi: 10.3389/fonc.2023.1121401. eCollection 2023. Front Oncol. 2023. PMID: 36937386 Free PMC article. Review.
References
-
- Kamat A.M., Hegarty P.K., Gee J.R., Clark P.E., Svatek R.S., Hegarty N., Shariat S.F., Xylinas E., Schmitz-Dräger B.J., Lotan Y., et al. International consultation on urologic disease—European association of urology consultation on bladder cancer 2012. ICUD-EAU international consultation on bladder cancer 2012: Screening, diagnosis, and molecular markers. Eur Urol. 2013;63:4–15. doi: 10.1016/j.eururo.2012.09.057. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

