Transport and Toxicity of Methylmercury-Cysteine in Cultured BeWo Cells
- PMID: 35008820
- PMCID: PMC8745507
- DOI: 10.3390/ijms23010394
Transport and Toxicity of Methylmercury-Cysteine in Cultured BeWo Cells
Abstract
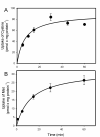
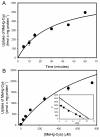
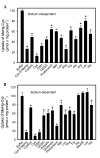
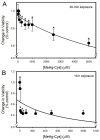
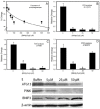
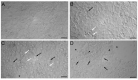
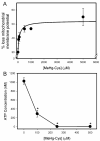
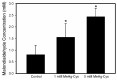
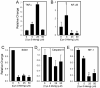
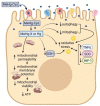
Mercury is a heavy metal toxicant that is prevalent throughout the environment. Organic forms of mercury, such as methylmercury (MeHg), can cross the placenta and can lead to lasting detrimental effects in the fetus. The toxicological effects of MeHg on the placenta itself have not been clearly defined. Therefore, the purpose of the current study was to assess the transport of MeHg into placental syncytiotrophoblasts and to characterize the mechanisms by which MeHg exerts its toxic effects. Cultured placental syncytiotrophoblasts (BeWo) were used for these studies. The transport of radioactive MeHg was measured to identify potential mechanisms involved in the uptake of this compound. The toxicological effects of MeHg on BeWo cells were determined by assessing visible pathological change, autophagy, mitochondrial viability, and oxidative stress. The findings of this study suggest that MeHg compounds are transported into BeWo cells primarily by sodium-independent amino acid carriers and organic anion transporters. The MeHg altered mitochondrial function and viability, decreased mitophagy and autophagy, and increased oxidative stress. Exposure to higher concentrations of MeHg inhibited the ability of cells to protect against MeHg-induced injury. The findings show that MeHg is directly toxic to syncytiotrophoblasts and may lead to disruptions in the fetal/maternal transfer of nutrients and wastes.
Keywords: autophagy; mercury; oxidative stress; placenta; syncytiotrophoblast; toxicology.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- ATSDR, Agency for Toxic Substances and Disease Registry, Toxicological Profile for Mercury . U.S. Department of Health and Human Services. Public Health Service, Centers for Disease Control; Atlanta, GA, USA: 2008. [(accessed on 21 July 2021)]. Available online: https://www.atsdr.cdc.gov/toxprofiles/tp46.pdf.
-
- Wickliffe J.K., Lichtveld M.Y., Zijlmans C.W., MacDonald-Ottevanger S., Shafer M., Dahman C., Harville E.W., Drury S., Landburg G., Ouboter P. Exposure to total and methylmercury among pregnant women in Suriname: Sources and public health implications. J. Expo. Sci. Environ. Epidemiol. 2020;31:117–125. doi: 10.1038/s41370-020-0233-3. - DOI - PMC - PubMed
-
- Llorente Ballesteros M.T., Garcia Barrado B., Navarro Serrano I., Izquierdo Alvarez S., Del Pueyo Garcia Anaya M., Gonzalez Munoz M.J. Evaluation of blood mercury and serum selenium levels in the pregnant population of the Community of Madrid, Spain. J. Trace Elements Med. Biol. 2020;57:60–67. doi: 10.1016/j.jtemb.2019.09.008. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

