Two Novel Dimorphism-Related Virulence Factors of Zymoseptoria tritici Identified Using Agrobacterium-Mediated Insertional Mutagenesis
- PMID: 35008825
- PMCID: PMC8745584
- DOI: 10.3390/ijms23010400
Two Novel Dimorphism-Related Virulence Factors of Zymoseptoria tritici Identified Using Agrobacterium-Mediated Insertional Mutagenesis
Abstract
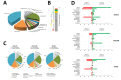
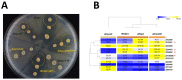
Diseases caused by dimorphic phytopathogenic and systemic dimorphic fungi have markedly increased in prevalence in the last decades, and understanding the morphogenic transition to the virulent state might yield novel means of controlling dimorphic fungi. The dimorphic fungus Z. tritici causes significant economic impact on wheat production, and yet the regulation of the dimorphic switch, a key first step in successful plant colonization, is still largely unexplored in this fungus. The fungus is amenable to suppression by fungicides at this switch point, and the identification of the factors controlling the dimorphic switch provides a potential source of novel targets to control Septoria tritici blotch (STB). Inhibition of the dimorphic switch can potentially prevent penetration and avoid any damage to the host plant. The aim of the current work was to unveil genetic determinants of the dimorphic transition in Z. tritici by using a forward genetics strategy. Using this approach, we unveiled two novel factors involved in the switch to the pathogenic state and used reverse genetics and complementation to confirm the role of the novel virulence factors and further gained insight into the role of these genes, using transcriptome analysis via RNA-Seq. The transcriptomes generated potentially contain key determinants of the dimorphic transition.
Keywords: RNA-Seq; Zymoseptoria tritici; dimorphic switch; forward genetics; fungal dimorphism; melanin; mycelium; pseudohyphal growth; reverse genetics; transcriptomic analysis.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures








Similar articles
-
Comparative transcriptomic analyses of Zymoseptoria tritici strains show complex lifestyle transitions and intraspecific variability in transcription profiles.Mol Plant Pathol. 2016 Aug;17(6):845-59. doi: 10.1111/mpp.12333. Epub 2016 Feb 8. Mol Plant Pathol. 2016. PMID: 26610174 Free PMC article.
-
The transcription factor Zt107320 affects the dimorphic switch, growth and virulence of the fungal wheat pathogen Zymoseptoria tritici.Mol Plant Pathol. 2020 Jan;21(1):124-138. doi: 10.1111/mpp.12886. Epub 2019 Nov 8. Mol Plant Pathol. 2020. PMID: 31702117 Free PMC article.
-
Identification of factors involved in dimorphism and pathogenicity of Zymoseptoria tritici.PLoS One. 2017 Aug 22;12(8):e0183065. doi: 10.1371/journal.pone.0183065. eCollection 2017. PLoS One. 2017. PMID: 28829795 Free PMC article.
-
How Knowledge of Pathogen Population Biology Informs Management of Septoria Tritici Blotch.Phytopathology. 2016 Sep;106(9):948-55. doi: 10.1094/PHYTO-03-16-0131-RVW. Epub 2016 Jul 27. Phytopathology. 2016. PMID: 27111799 Review.
-
Cell biology of Zymoseptoria tritici: Pathogen cell organization and wheat infection.Fungal Genet Biol. 2015 Jun;79:17-23. doi: 10.1016/j.fgb.2015.04.002. Fungal Genet Biol. 2015. PMID: 26092785 Free PMC article. Review.
Cited by
-
Use of chitin:DNA ratio to assess growth form in fungal cells.BMC Biol. 2024 Jan 17;22(1):10. doi: 10.1186/s12915-024-01815-2. BMC Biol. 2024. PMID: 38233847 Free PMC article.
-
Quantitative and qualitative plant-pathogen interactions call upon similar pathogenicity genes with a spectrum of effects.Front Plant Sci. 2023 May 10;14:1128546. doi: 10.3389/fpls.2023.1128546. eCollection 2023. Front Plant Sci. 2023. PMID: 37235026 Free PMC article.
-
Defense Pathways of Wheat Plants Inoculated with Zymoseptoria tritici under NaCl Stress Conditions: An Overview.Life (Basel). 2024 May 20;14(5):648. doi: 10.3390/life14050648. Life (Basel). 2024. PMID: 38792668 Free PMC article. Review.
-
Fungal plant pathogen "mutagenomics" reveals tagged and untagged mutations in Zymoseptoria tritici and identifies SSK2 as key morphogenesis and stress-responsive virulence factor.Front Plant Sci. 2023 May 3;14:1140824. doi: 10.3389/fpls.2023.1140824. eCollection 2023. Front Plant Sci. 2023. PMID: 37206970 Free PMC article.
References
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources

