Clinical Significance of Stem Cell Biomarkers EpCAM, LGR5 and LGR4 mRNA Levels in Lymph Nodes of Colon Cancer Patients
- PMID: 35008827
- PMCID: PMC8745090
- DOI: 10.3390/ijms23010403
Clinical Significance of Stem Cell Biomarkers EpCAM, LGR5 and LGR4 mRNA Levels in Lymph Nodes of Colon Cancer Patients
Abstract
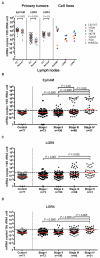
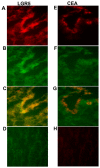
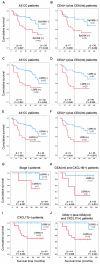
The significance of cancer stem cells (CSCs) in initiation and progression of colon cancer (CC) has been established. In this study, we investigated the utility of measuring mRNA expression levels of CSC markers EpCAM, LGR5 and LGR4 for predicting survival outcome in surgically treated CC patients. Expression levels were determined in 5 CC cell lines, 66 primary CC tumors and 382 regional lymph nodes of 121 CC patients. Prognostic relevance was determined using Kaplan-Meier survival and Cox regression analyses. CC patients with lymph nodes expressing high levels of EpCAM, LGR5 or LGR4 (higher than a clinical cutoff of 0.07, 0.06 and 2.558 mRNA copies/18S rRNA unit, respectively) had a decreased mean survival time of 32 months for EpCAM and 42 months for both LGR5 and LGR4 at a 12-year follow-up (p = 0.022, p = 0.005 and p = 0.011, respectively). Additional patients at risk for recurrence were detected when LGR5 was combined with the biomarkers CXCL17 or CEA plus CXCL16. In conclusion, the study underscores LGR5 as a particularly useful prognostic biomarker and illustrates the strength of combining biomarkers detecting different subpopulations of cancer cells and/or cells in the tumor microenvironment for predicting recurrence.
Keywords: CEA; CXCL16; CXCL17; EpCAM; LGR4; LGR5; colon cancer; prognosis; qRT-PCR; regional lymph nodes; stem cell markers.
Conflict of interest statement
The authors declare no competing interests.
Figures

References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

