Curcumin as an Epigenetic Therapeutic Agent in Myelodysplastic Syndromes (MDS)
- PMID: 35008835
- PMCID: PMC8745143
- DOI: 10.3390/ijms23010411
Curcumin as an Epigenetic Therapeutic Agent in Myelodysplastic Syndromes (MDS)
Abstract
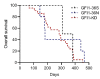
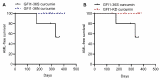
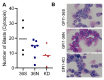
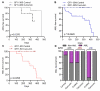
Growth Factor Independence 1 (GFI1) is a transcription factor with an important role in the regulation of development of myeloid and lymphoid cell lineages and was implicated in the development of myelodysplastic syndrome (MDS) and acute myeloid leukaemia (AML). Reduced expression of GFI1 or presence of the GFI1-36N (serine replaced with asparagine) variant leads to epigenetic changes in human and murine AML blasts and accelerated the development of leukaemia in a murine model of human MDS and AML. We and other groups previously showed that the GFI1-36N allele or reduced expression of GFI1 in human AML blasts is associated with an inferior prognosis. Using GFI1-36S, -36N -KD, NUP98-HOXD13-tg mice and curcumin (a natural histone acetyltransferase inhibitor (HATi)), we now demonstrate that expansion of GFI1-36N or -KD, NUP98-HODXD13 leukaemic cells can be delayed. Curcumin treatment significantly reduced AML progression in GFI1-36N or -KD mice and prolonged AML-free survival. Of note, curcumin treatment had no effect in GFI1-36S, NUP98-HODXD13 expressing mice. On a molecular level, curcumin treatment negatively affected open chromatin structure in the GFI1-36N or -KD haematopoietic cells but not GFI1-36S cells. Taken together, our study thus identified a therapeutic role for curcumin treatment in the treatment of AML patients (homo or heterozygous for GFI1-36N or reduced GFI1 expression) and possibly improved therapy outcome.
Keywords: GFI1; acute myeloid leukaemia; curcumin; histone acetyltransferase inhibitor.
Conflict of interest statement
The authors declare that there is no conflict of interest.
Figures


Similar articles
-
Epigenetic therapy as a novel approach for GFI136N-associated murine/human AML.Exp Hematol. 2016 Aug;44(8):713-726.e14. doi: 10.1016/j.exphem.2016.05.004. Epub 2016 May 20. Exp Hematol. 2016. PMID: 27216773
-
Germ line variant GFI1-36N affects DNA repair and sensitizes AML cells to DNA damage and repair therapy.Blood. 2023 Dec 21;142(25):2175-2191. doi: 10.1182/blood.2022015752. Blood. 2023. PMID: 37756525 Free PMC article.
-
GFI1(36N) as a therapeutic and prognostic marker for myelodysplastic syndrome.Exp Hematol. 2016 Jul;44(7):590-595.e1. doi: 10.1016/j.exphem.2016.04.001. Epub 2016 Apr 11. Exp Hematol. 2016. PMID: 27080012 Free PMC article.
-
Role of GFI1 in Epigenetic Regulation of MDS and AML Pathogenesis: Mechanisms and Therapeutic Implications.Front Oncol. 2019 Aug 27;9:824. doi: 10.3389/fonc.2019.00824. eCollection 2019. Front Oncol. 2019. PMID: 31508375 Free PMC article. Review.
-
Epigenetic regulation in myelodysplastic syndromes: implications for therapy.Expert Opin Investig Drugs. 2011 Apr;20(4):465-93. doi: 10.1517/13543784.2011.559164. Epub 2011 Mar 8. Expert Opin Investig Drugs. 2011. PMID: 21381982 Review.
Cited by
-
The Role of Epigenetic Modifications in Human Cancers and the Use of Natural Compounds as Epidrugs: Mechanistic Pathways and Pharmacodynamic Actions.Biomolecules. 2022 Feb 25;12(3):367. doi: 10.3390/biom12030367. Biomolecules. 2022. PMID: 35327559 Free PMC article. Review.
-
Database of space life investigations and information on spaceflight plant biology.Planta. 2023 Aug 1;258(3):58. doi: 10.1007/s00425-023-04213-0. Planta. 2023. PMID: 37528331
-
The dynamics of DNA methylation, histone methylation and acetylation during oocyte aging in mammalian species and possible interventions to regulate them.J Assist Reprod Genet. 2025 Jul 14. doi: 10.1007/s10815-025-03577-4. Online ahead of print. J Assist Reprod Genet. 2025. PMID: 40653579 Review.
-
LL-37 regulates odontogenic differentiation of dental pulp stem cells in an inflammatory microenvironment.Stem Cell Res Ther. 2024 Dec 18;15(1):469. doi: 10.1186/s13287-024-04075-7. Stem Cell Res Ther. 2024. PMID: 39696668 Free PMC article.
-
Tyrosinase enzyme purification and immobilization from Pseudomonas sp. EG22 using cellulose coated magnetic nanoparticles: characterization and application in melanin production.World J Microbiol Biotechnol. 2023 Nov 10;40(1):10. doi: 10.1007/s11274-023-03796-w. World J Microbiol Biotechnol. 2023. PMID: 37947912 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

