Antifungal Activity of N-(4-Halobenzyl)amides against Candida spp. and Molecular Modeling Studies
- PMID: 35008845
- PMCID: PMC8745543
- DOI: 10.3390/ijms23010419
Antifungal Activity of N-(4-Halobenzyl)amides against Candida spp. and Molecular Modeling Studies
Abstract
Fungal infections remain a high-incidence worldwide health problem that is aggravated by limited therapeutic options and the emergence of drug-resistant strains. Cinnamic and benzoic acid amides have previously shown bioactivity against different species belonging to the Candida genus. Here, 20 cinnamic and benzoic acid amides were synthesized and tested for inhibition of C. krusei ATCC 14243 and C. parapsilosis ATCC 22019. Five compounds inhibited the Candida strains tested, with compound 16 (MIC = 7.8 µg/mL) producing stronger antifungal activity than fluconazole (MIC = 16 µg/mL) against C. krusei ATCC 14243. It was also tested against eight Candida strains, including five clinical strains resistant to fluconazole, and showed an inhibitory effect against all strains tested (MIC = 85.3-341.3 µg/mL). The MIC value against C. krusei ATCC 6258 was 85.3 mcg/mL, while against C. krusei ATCC 14243, it was 10.9 times smaller. This strain had greater sensitivity to the antifungal action of compound 16. The inhibition of C. krusei ATCC 14243 and C. parapsilosis ATCC 22019 was also achieved by compounds 2, 9, 12, 14 and 15. Computational experiments combining target fishing, molecular docking and molecular dynamics simulations were performed to study the potential mechanism of action of compound 16 against C. krusei. From these, a multi-target mechanism of action is proposed for this compound that involves proteins related to critical cellular processes such as the redox balance, kinases-mediated signaling, protein folding and cell wall synthesis. The modeling results might guide future experiments focusing on the wet-lab investigation of the mechanism of action of this series of compounds, as well as on the optimization of their inhibitory potency.
Keywords: Candida auris; anticandidal drugs; antimicrobial activity; benzoic acid; candidiasis; cinnamic acid; fungi; molecular docking; natural products; plants.
Conflict of interest statement
No conflicts of interest are associated with this work.
Figures
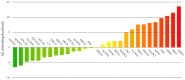
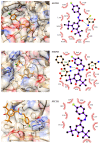



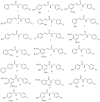
References
-
- Barbedo L.S., Sgarbi D.B.G. Candidíase. DST J. Bras. Doenças Sex Transm. 2010;22:22–38.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

