The YAP/TAZ Signaling Pathway in the Tumor Microenvironment and Carcinogenesis: Current Knowledge and Therapeutic Promises
- PMID: 35008857
- PMCID: PMC8745604
- DOI: 10.3390/ijms23010430
The YAP/TAZ Signaling Pathway in the Tumor Microenvironment and Carcinogenesis: Current Knowledge and Therapeutic Promises
Abstract
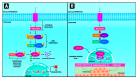
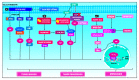
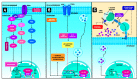
The yes-associated protein (YAP) and the transcriptional coactivator with PDZ-binding motif (TAZ) are transcriptional coactivators, members of the Hippo signaling pathway, which play a critical role in cell growth regulation, embryonic development, regeneration, proliferation, and cancer origin and progression. The mechanism involves the nuclear binding of the un-phosphorylated YAP/TAZ complex to release the transcriptional enhanced associate domain (TEAD) from its repressors. The active ternary complex is responsible for the aforementioned biological effects. Overexpression of YAP/TAZ has been reported in cancer stem cells and tumor resistance. The resistance involves chemotherapy, targeted therapy, and immunotherapy. This review provides an overview of YAP/TAZ pathways' role in carcinogenesis and tumor microenvironment. Potential therapeutic alternatives are also discussed.
Keywords: Hippo signaling pathway; TEAD; YAP/TAZ; carcinogenesis; cell proliferation; chemoresistance; drug resistance; immunotherapy; neoplastic stem cells; tumor microenvironment.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- León J.D., Pareja A. Inmunología del cáncer II: Bases moleculares y celulares de la carcinogénesis. Horiz. Médico (Lima) 2019;19:84–92. doi: 10.24265/horizmed.2019.v19n2.11. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

